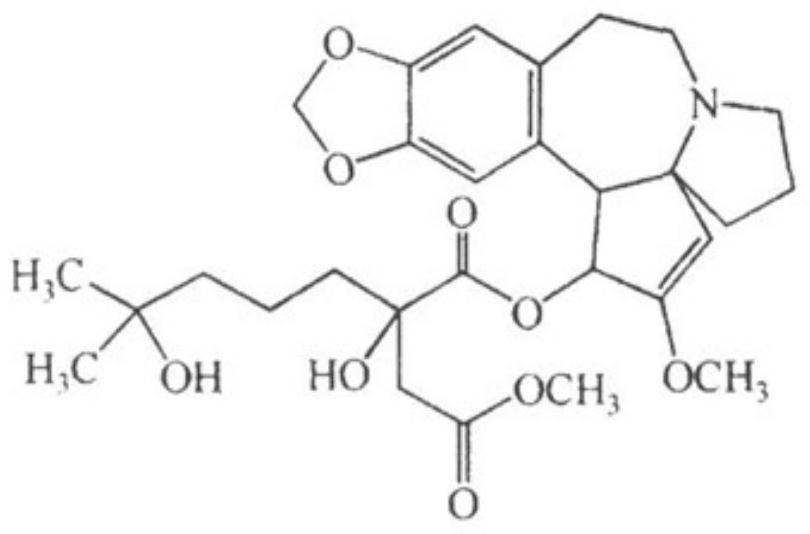
A plant source preparation for antiviral infection of livestock and poultry
An anti-virus, livestock and poultry technology, applied in the field of plant-derived preparations for anti-viral infection of livestock and poultry, can solve problems such as unclear anti-viral effect, and achieve the effect of broad-spectrum and high-efficiency anti-viral activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0031] 1. Preparation of cells
[0032] DMEM medium containing 10% fetal bovine serum and 100U penicillin and streptomycin was used for HeLa, Vero, PK15 and Marc145 cell passage, and DMEM medium containing 2% fetal bovine serum and 100U penicillin streptomycin was used as maintenance medium.
[0033] NDV infected HeLa cells, VSV, HSV-1, PEDV, PRV infected Vero cells, TGEV, FMDV infected PK15 cells, PRRSV infected Marc145 cells.
[0034] 2. Virus proliferation
[0035] Proliferation of HSV-1, NDV, VSV, and PRV cytotoxicity: Inoculate the virus stock solution according to the amount of 1 / 10 of the cell flask culture medium, and wait for the cytopathic changes (such as NDV to form large syncytia) to reach 75% (usually 48 hours) to use repeatedly Harvest the virus by freezing and thawing, that is, store the cell bottle at -80°C and store it at room temperature until it partially thaws. The virus was released from the cells and frozen at -80°C.
[0036] Proliferation of NDV chic...
Embodiment 1
[0043] Embodiment 1 The influence of HHT on the virulence of VSV virus
[0044] Infect HeLa cells with 0.5MOI of VSV, add different concentrations of HHT and Ribavirin (Ribavirin) to the cell culture medium after 1.5h, freeze and thaw three times after infecting 24h, measure virus virulence, the results show that compared with Ribavirin, the effective antiviral concentration of HHT is 1000 times less ( figure 2 A).
[0045] HeLa cells were infected with 1 MOI of VSV, 1.5 hours later, different concentrations of HHT were added to the cell culture medium, and the cells were collected after 36 hours of virus infection. The expression of viral protein G protein was detected by immunoblotting, and the results showed that HHT significantly inhibited the expression of G protein in a dose-dependent manner. HeLa cells were infected with 0.5 MOI of VSV, and 50nM HHT was added after 1.5h, and the cell samples were collected at 8h, 24h, and 36h to detect the expression of viral protein...
Embodiment 2
[0047] Example 2 Effect of HHT on NDV virus virulence
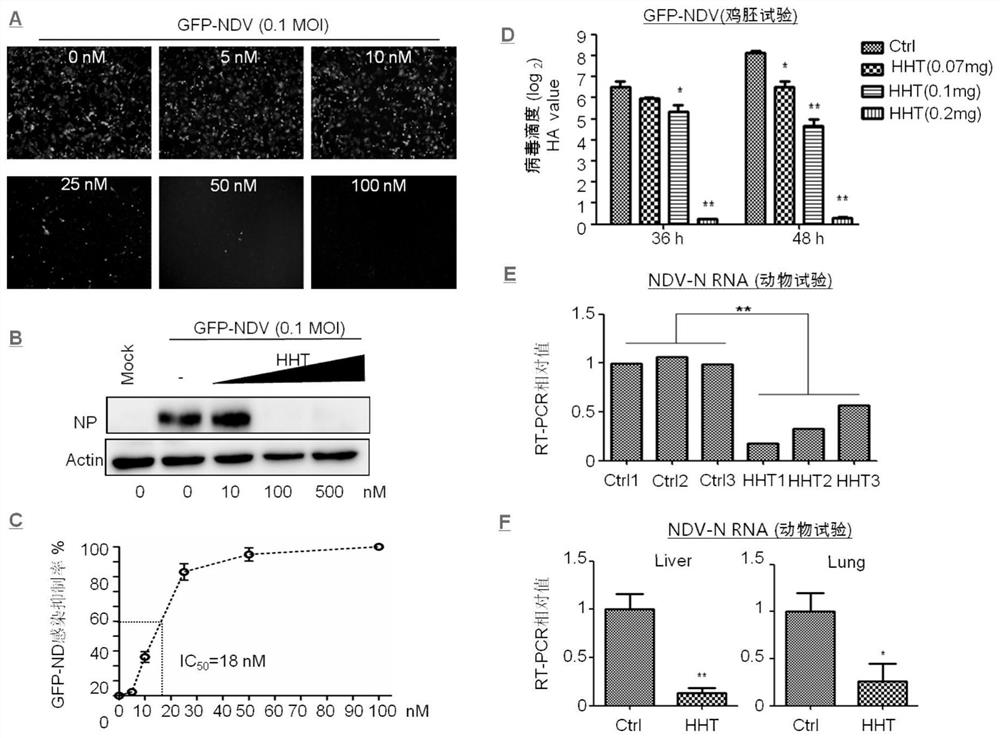
[0048]Infect HeLa cells with 0.1 MOI of GFP-NDV (green fluorescent protein-labeled NDV), add different concentrations of HHT to the cell culture medium after 1.5 h, and the concentration of HHT from low to high is 5, 10, 25, 50 , 100 nM. After virus infection 24h, observe in fluorescent microscope Olympus IX73, image with CellSens software, the result shows that, HHT significantly inhibits GFP-NDV infection and is dose-dependent ( image 3 A).
[0049] HeLa cells were infected with 0.1 MOI of GFP-NDV. After 1.5 hours, different concentrations of HHT were added to the cell culture medium. After 36 hours of virus infection, the cells were collected, and the expression of viral protein NP was detected by Western blotting. The results showed that HHT significantly inhibited the expression of NDV protein and in a dose-dependent manner ( image 3 B).
[0050] HeLa cells were infected with 0.1 MOI of GFP-NDV. After 1.5 hours...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com