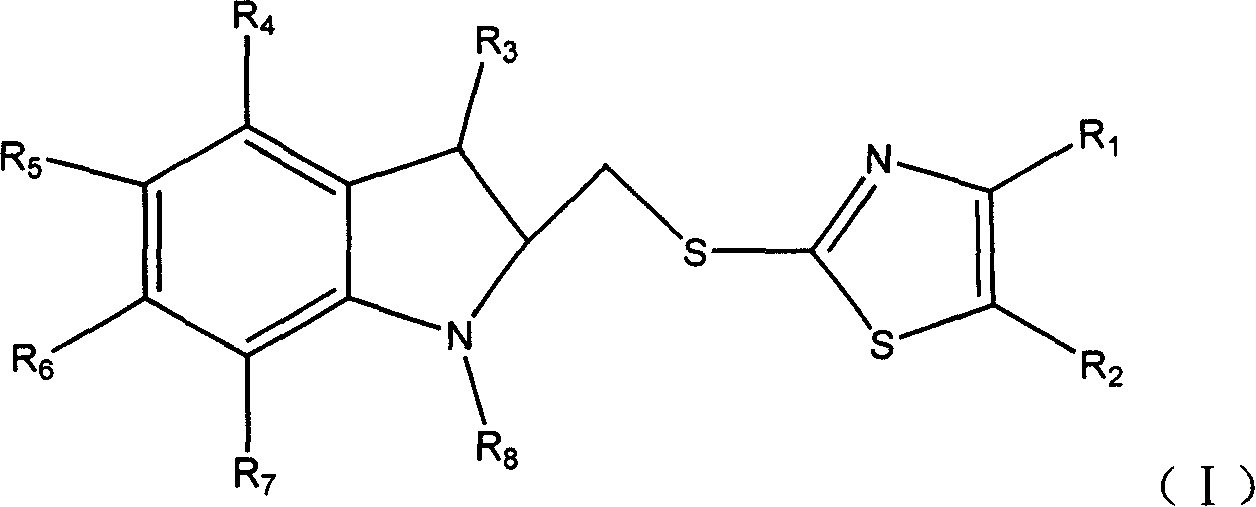
Novel compound with antiviral activity
A compound, methyl technology, applied in the field of medicine, can solve the problems of narrow activity range, increased drug resistance, high price, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
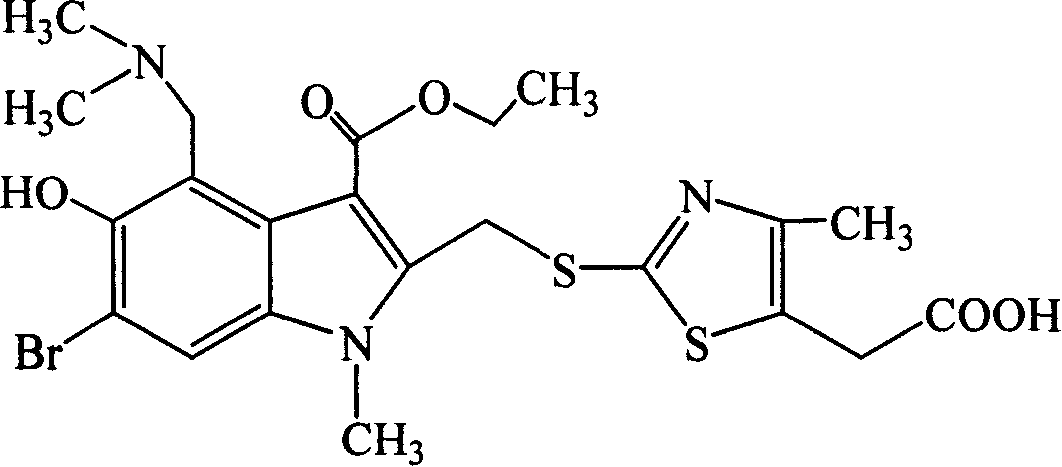
[0185] Example 1 6-bromo-4-dimethylaminomethyl-5-hydroxyl-1-methyl-2-(4-methyl-2-thiomethyl-5-carboxymethyl-thiazole)-1H- Preparation of ethyl indole-3-carboxylate hydrochloride
[0186] (1) 6-Bromo-5-hydroxy-1-methyl-2-(4-methyl-2-thiomethyl-5-carboxymethyl-thiazole)-1H-indole-3-carboxylic acid ethyl ester preparation of
[0187] In the dry reaction bottle, add methanol 300ml, potassium hydroxide 13g (0.23mol), after stirring and dissolving, add 27g (0.23mol) 4-methyl-2-thiomethyl-5-carboxymethyl-thiazole in batches, Stir at room temperature for 10 min. Then 90 g (0.23 mol) of ethyl 5-acetoxy-6-bromo-2-bromomethyl-1-methyl-1H-indole-3-carboxylate was added, stirred for 12 hours, and the reaction was completed. Pour the reaction solution into ice water under stirring, adjust the pH to about 2 with dilute hydrochloric acid, precipitate out, filter with suction, wash the filter cake with water and dry to obtain 74.6 g of light yellow solid, namely 6-bromo-5-hydroxy-1-methy...
Embodiment 2
[0193] Example 2 6-bromo-4-dimethylaminomethyl-5-hydroxy-1-methyl-2-(2-thiomethyl-thiazole)-1H-indole-3-carboxylic acid ethyl ester Preparation of hydrochloride
[0194] (1) Preparation of 6-bromo-5-hydroxyl-1-methyl-2-(2-thiomethyl-thiazole)-1H-indole-3-carboxylic acid ethyl ester
[0195] In the dry reaction bottle, add 300ml of methanol and 13g (0.23mol) of potassium hydroxide, stir and dissolve, add 27g (0.23mol) of 2-mercaptothiazole in batches, and stir at room temperature for 10min. Then 90 g (0.23 mol) of ethyl 5-acetoxy-6-bromo-2-bromomethyl-1-methyl-1H-indole-3-carboxylate was added, stirred for 12 hours, and the reaction was completed. Pour the reaction solution into ice water under stirring, adjust the pH to about 2 with dilute hydrochloric acid, precipitate out, filter with suction, wash the filter cake with water and dry to obtain 74.6 g of light yellow solid, namely 6-bromo-5-hydroxy-1-methyl Ethyl-2-(2-thiomethyl-thiazole)-1H-indole-3-carboxylate, yield: 7...
Embodiment 3
[0201] Example 3 6-bromo-4-dimethylaminomethyl-5-hydroxyl-1-methyl-2-(4-methyl-2-thiomethyl-thiazole)-1H-indole-3- Preparation of ethyl carboxylate hydrochloride
[0202] (1) Preparation of 6-bromo-5-hydroxyl-1-methyl-2-(4-methyl-2-thiomethyl-thiazole)-1H-indole-3-carboxylic acid ethyl ester
[0203] In a dry reaction flask, add 300ml of methanol and 13g (0.23mol) of potassium hydroxide, stir to dissolve, add 27g (0.23mol) of 4-methyl-2-thiomethyl-thiazole in batches, and stir at room temperature for 10min. Then 90 g (0.23 mol) of ethyl 5-acetoxy-6-bromo-2-bromomethyl-1-methyl-1H-indole-3-carboxylate was added, stirred for 12 hours, and the reaction was completed. Pour the reaction solution into ice water under stirring, adjust the pH to about 2 with dilute hydrochloric acid, precipitate out, filter with suction, wash the filter cake with water and dry to obtain 74.6 g of light yellow solid, namely 6-bromo-5-hydroxy-1-methyl Ethyl-2-(4-methyl-2-thiomethyl-thiazole)-1H-ind...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com