Plastic lens
A plastic lens and lens technology, applied in glasses/goggles, optics, instruments, etc., can solve problems such as discoloration, inability to obtain sufficient effects, and lack of solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
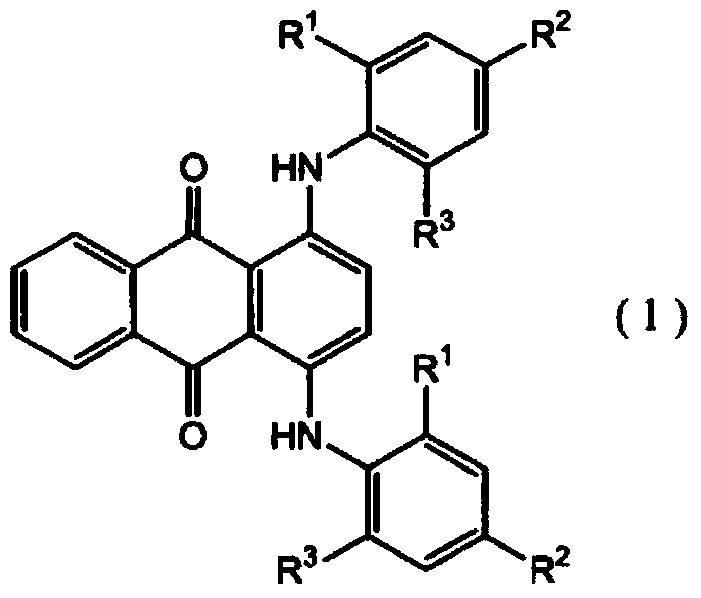
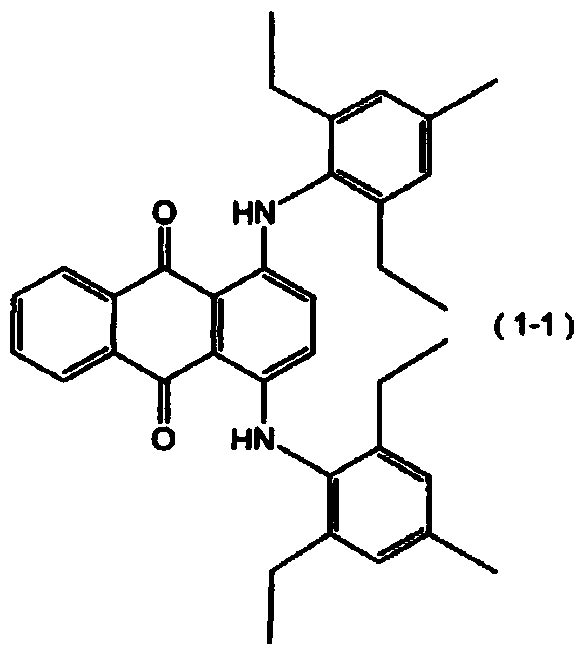
[0051] 22.88 g of 4,4'-diphenylmethane diisocyanate and 25.09 g of 1,6-hexamethylene diisocyanate were placed in a 500 ml eggplant-shaped bottle, and JP-506H (trade name, Chengbei Chemical Co., Ltd.) was added as a mold release agent. Industry Co., Ltd.) 0.20 g, 0.06 g of dimethyl tin dichloride as a polymerization catalyst, 1.00 g of SEESORB701 (trade name, manufactured by Shipro Chemical Co., Ltd.) as an ultraviolet absorber, and the following formula (1-1) The indicated compound 1,4-bis[(2,6-diethyl-4-methylphenyl)amino]anthracene-9,10-dione 0.8 mass ppm was continuously stirred at 50°C under nitrogen purge for 30 minute. When these were completely dissolved, 52.03 g of pentaerythritol tetrakis(2-mercaptoacetate) was blended and stirred under reduced pressure at 1.3 kPa for 20 minutes to prepare a mixture.
[0052] The mixture was injected into a lens mold with a central wall thickness of 2 mm after passing through a 1.0 μm PTFE membrane filter, and polymerized by a temper...
Embodiment 2
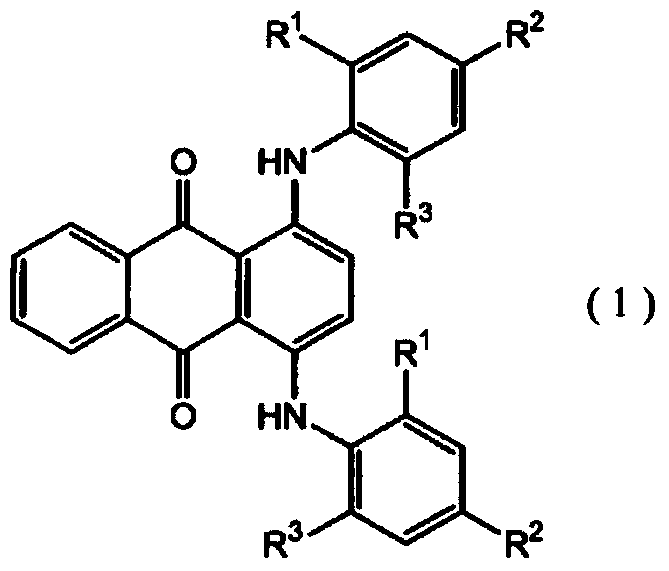
[0057] In Example 1, the compound 1,4-bis[(2,4,6-trimethylphenyl)amino]anthracene-9,10-dione represented by the following formula (1-2) was used instead of 1 , 4-bis[(2,6-diethyl-4-methylphenyl)amino]anthracene-9,10-dione, and a lens was obtained by the same procedure.
[0058] The obtained lens was colorless and transparent without substantially yellowing, and had a YI value of 1.41.
[0059] [chemical formula 4]
[0060]
Embodiment 3
[0080] Put 22.25 g of 4,4'-diphenylmethane diisocyanate, 15.74 g of 1,6-hexamethylene diisocyanate, and 11.44 g of isophorone diisocyanate into a 500 ml eggplant-shaped bottle, and add 0.20 g of JP-506H (trade name, manufactured by Johoku Chemical Industry Co., Ltd.), 0.06 g of dimethyl tin dichloride as a polymerization catalyst, 1.00 g of SEESORB701 (trade name, manufactured by Shipro Chemical Industry Co., Ltd.) as an ultraviolet absorber, 1.00 g of 2,6-di-tert-butyl-p-cresol as an antioxidant, the compound 1,4-bis[(2,6-diethyl-4-methylbenzene) represented by the above formula (1-1) base) amino] anthracene-9,10-dione 0.4 mass ppm, and stirred continuously for 30 minutes at 50° C. under nitrogen purge. When these were completely dissolved, 50.58 g of pentaerythritol tetrakis(2-mercaptoacetate) was blended and stirred under reduced pressure at 1.3 kPa for 20 minutes to obtain a mixture.
[0081] The mixture was poured into a lens mold with a central wall thickness of 2 mm af...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com