Synthesis method of alpha-cyan-4-hydroxycinnamic acids modified silicon-containing matrix
A technology of hydroxycinnamic acid and synthesis method, applied in the field of synthesis of nano-silicon materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach 1
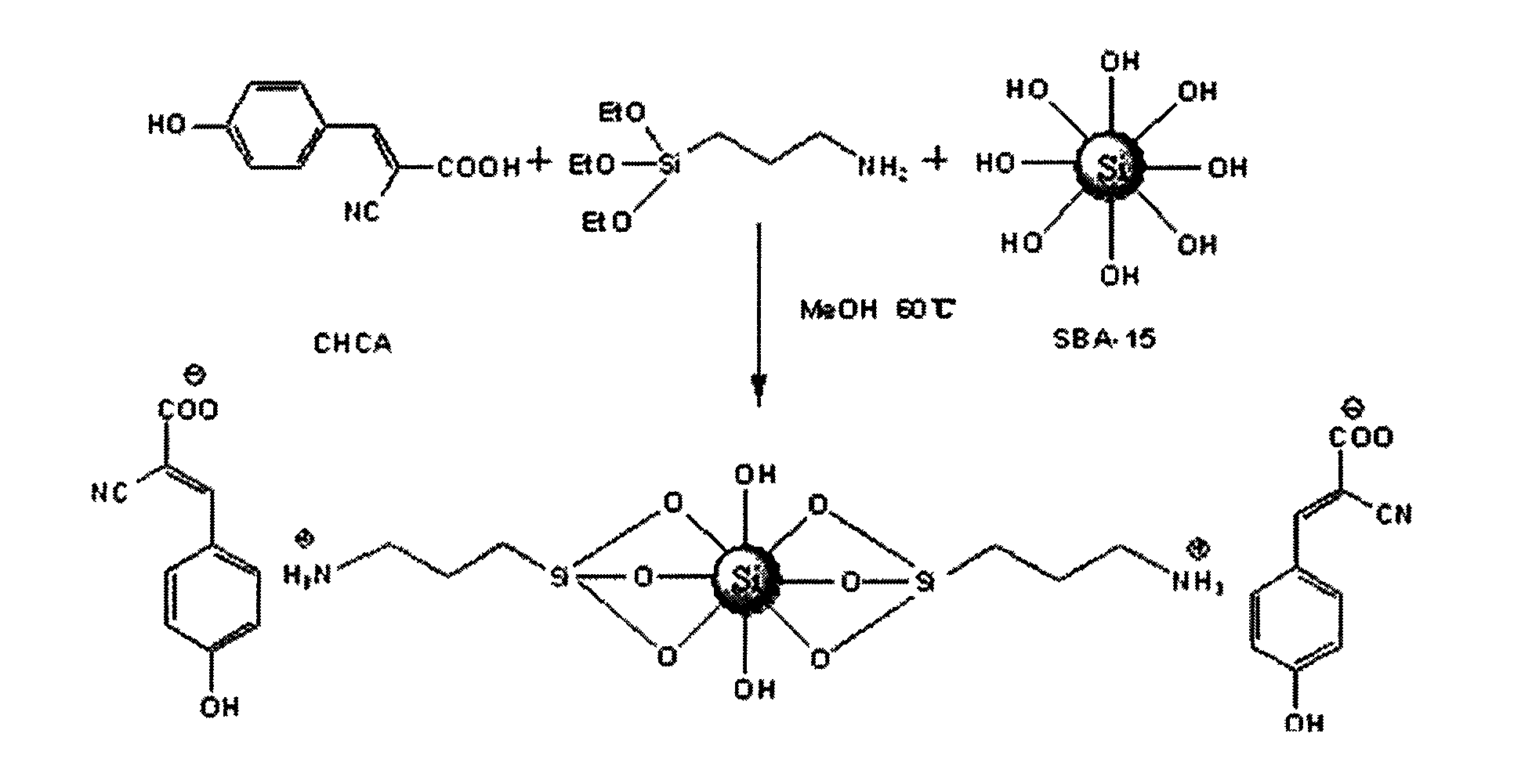
[0023] (1) Accurately weigh 1.0 mmol of CHCA in a 100 ml three-necked flask, add 30 ml of methanol, and stir for 15 minutes under nitrogen protection at room temperature, then add 1.0 mmol of 3-APTES solution dropwise, and continue stirring for 1 hour;
[0024] (2) Add 0.5g SBA-15, stir to make the system evenly mixed, then heat up to 60°C and reflux for 12h;
[0025] (3) The reacted system was centrifuged, the supernatant was removed, the precipitate was shaken and washed 6 times with 40 ml of methanol, and vacuum-dried at 60° C. for 6 hours to obtain the product.
Embodiment approach 2
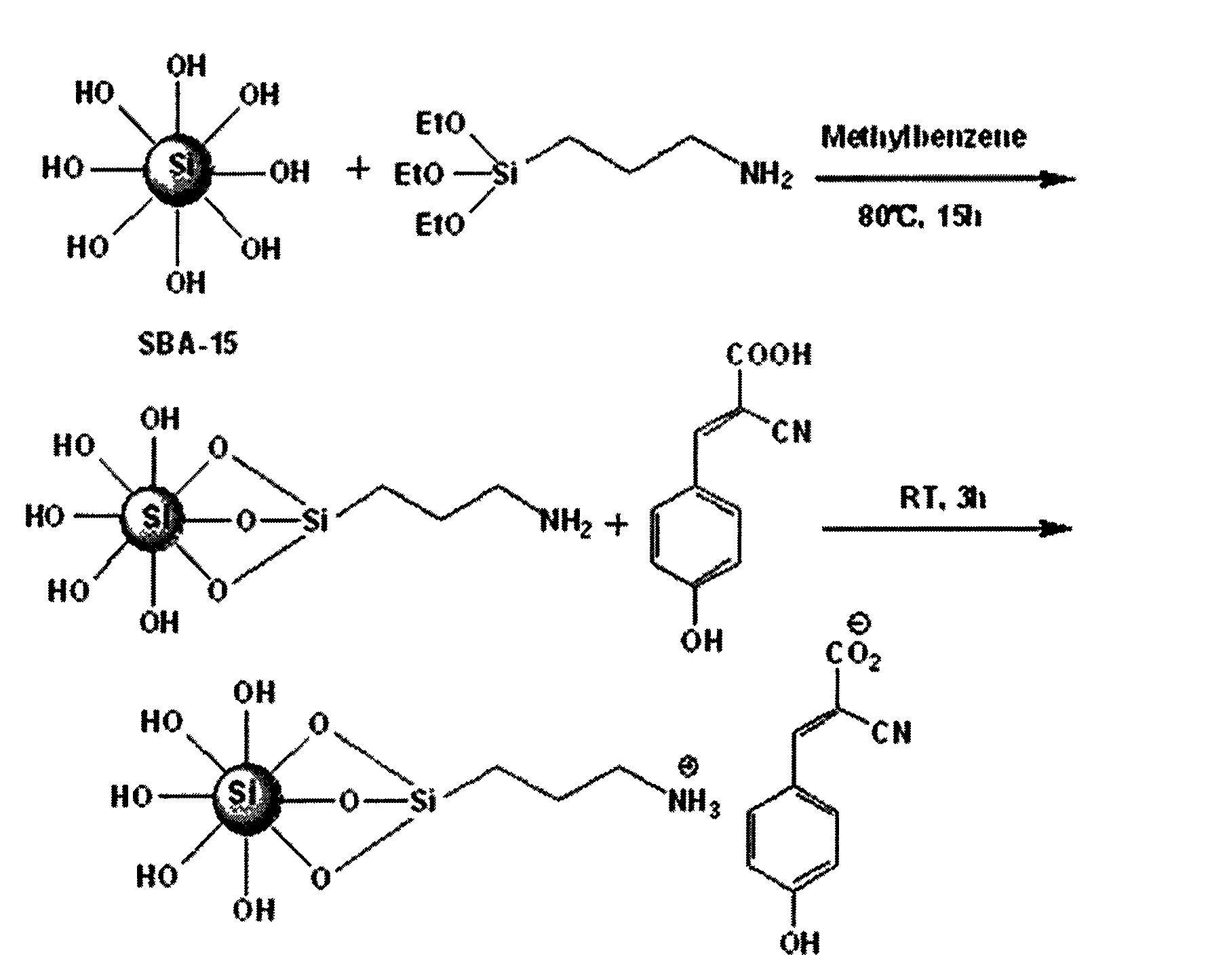
[0027] (1) Weigh 0.5g of dried SBA-15 into a 100ml three-necked flask, add 15ml of toluene, add 1.0mmol of 3-APTES dropwise, stir evenly, heat up to 80°C, and reflux for 15h under nitrogen protection;
[0028] (2) Suction filter the reacted system, wash with 40ml of toluene and isopropanol for 3 times, extract for 20min, and vacuum-dry the filter cake at 80°C for 10h to obtain amino-modified SBA-15;
[0029] (3) Weigh 1.0 mmol of CHCA into a 100 ml three-neck flask, add 30 ml of methanol, and stir under nitrogen protection at room temperature for 15 min, add the dried amino-modified SBA-15, and stir at room temperature for 3 h;
[0030] The reacted system was centrifuged to remove the supernatant, the precipitate was shaken and washed 6 times with 40 ml of methanol, and vacuum-dried at 60° C. for 6 hours to obtain the product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com