Preparation method of benzothiazole compound based on aminothiophenol cyclization
A technology of o-aminothiophenol and benzothiazole, which is applied in the field of preparation of pharmaceutical and chemical intermediates, and can solve problems such as high reaction temperature and complex reaction system
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1: Synthesis of 2-methylbenzothiazole (3a)
[0030]
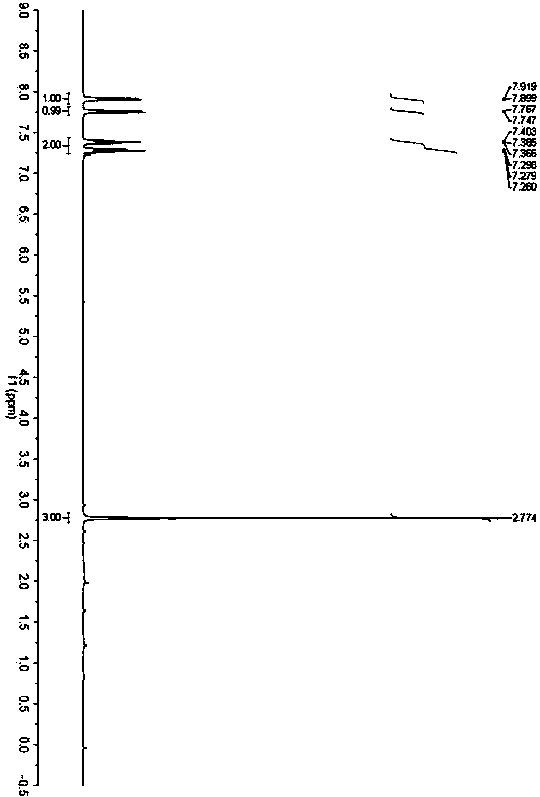
[0031] Accurately weigh o-aminothiophenol (62.5mg, 0.5mmol), acetylacetone (50.1mg, 0.5mmol) and benzoic acid (0.6mg, 0.005mmol), add them to a 25mL Schlenk bottle in sequence, and place at 20°C React in an oil bath for 24h. After the reaction was completed, the solvent was removed under reduced pressure, and petroleum ether / ethyl acetate was used as the eluent to separate through a silica gel column. The yield of 2-methylbenzothiazole was 92%. 1 H NMR (400MHz, CDCl 3 )δ2.78(s,3H)7.27-7.30(m,1H),7.37-7.41(m,1H),7.76(d,J=8.0Hz,1H),7.91(d,J=8.0Hz,1H) ; 13 C NMR (100MHz, CDCl 3 )δ17.7, 119.0, 119.9, 122.3, 123.5, 133.2, 150.9, 164.5.
Embodiment 2
[0032] Embodiment 2: Synthesis of 2-ethylbenzothiazole (3b)
[0033]
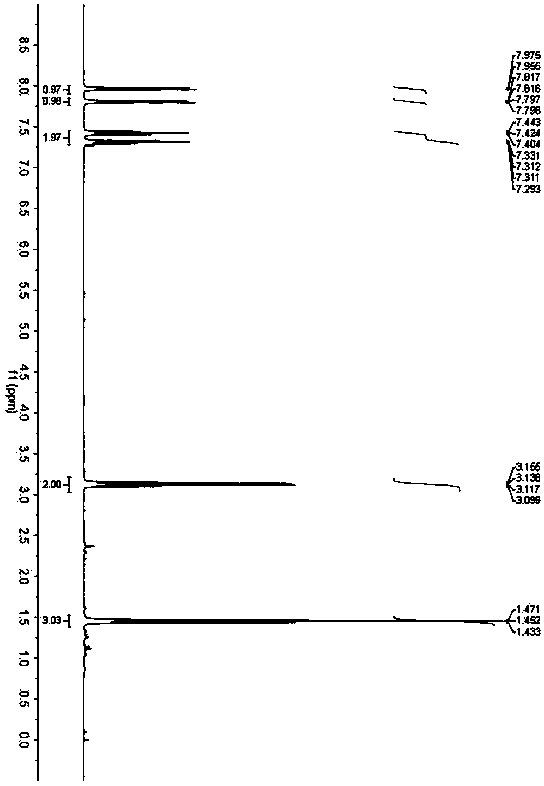
[0034] Accurately weigh o-aminothiophenol (62.5mg, 0.5mmol), 3,5-diheptanone (256.0mg, 2.0mmol) and benzoic acid (6.1mg, 0.05mmol), and add them to a 25mL Schlenk bottle in sequence , added 1,4-dioxane (3.0 mL), and placed in an oil bath at 200° C. for 36 h. After the reaction was completed, the solvent was removed under reduced pressure, and petroleum ether / ethyl acetate was used as the eluent to separate through a silica gel column. The yield of 2-ethylbenzothiazole was 90%. 1 H NMR (400MHz, CDCl 3 )δ1.45(t,J=7.6Hz,3H),3.13(q,J=7.6Hz,2H),7.31(dd,J=7.6,8.0Hz,1H),7.42(dd,J=7.6,8.0 Hz,1H),7.81(d,J=8.0Hz,1H),7.97(d,J=8.0Hz,1H); 13 C NMR (100MHz, CDCl 3 )δ13.7, 27.7, 121.4, 122.4, 124.5, 125.8, 135.0, 153.2, 173.4.
Embodiment 3
[0035] Embodiment 3: Synthesis of 2-ethyl-5-chlorobenzothiazole (3c)
[0036]
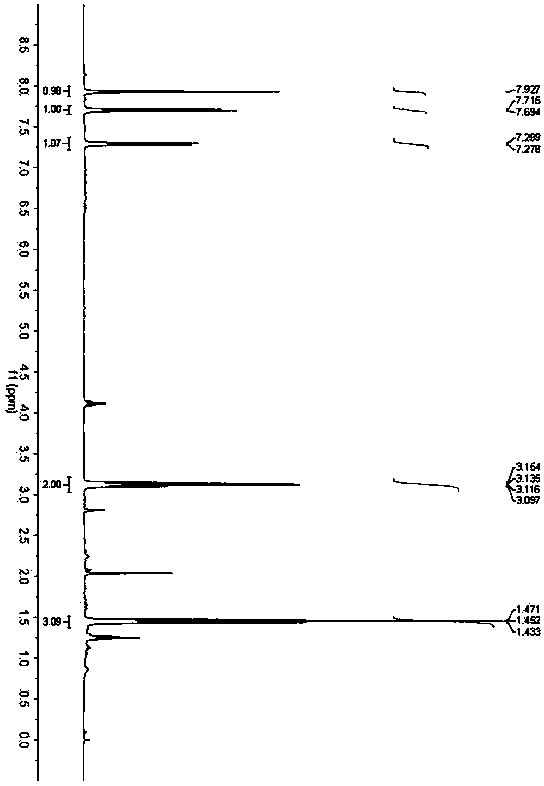
[0037] Accurately weigh 4-chloro-o-aminothiophenol (79.5mg, 0.5mmol), 3,5-diheptanone (64.0mg, 0.5mmol) and p-toluenesulfonic acid (9.5mg, 0.05mmol), and add them to Toluene (4.0 mL) was added into a 25 mL Schlenk bottle, and placed in an oil bath at 60° C. for 24 h. After the reaction was completed, the solvent was removed under reduced pressure, and petroleum ether / ethyl acetate was used as the eluent to separate through a silica gel column. The yield of 2-ethyl-5-chlorobenzothiazole was 85%. 1 H NMR (400MHz, CDCl 3 )δ1.45(t,J=7.6Hz,3H),3.13(q,J=7.6Hz,2H),7.29(d,J=8.4Hz,1H)7.70(d,J=8.4Hz,1H), 7.93(s,1H); 13 C NMR (100MHz, CDCl 3 )δ13.9, 28.1, 122.4, 122.6, 125.3, 132.1, 133.6, 154.4, 175.8.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com