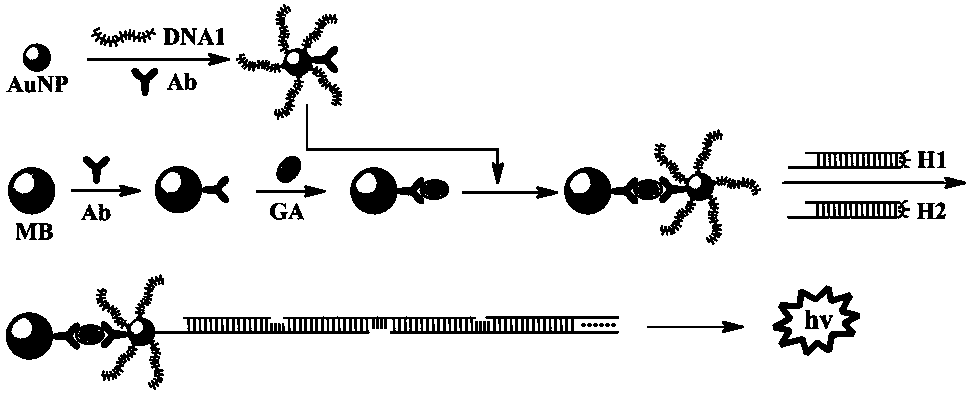
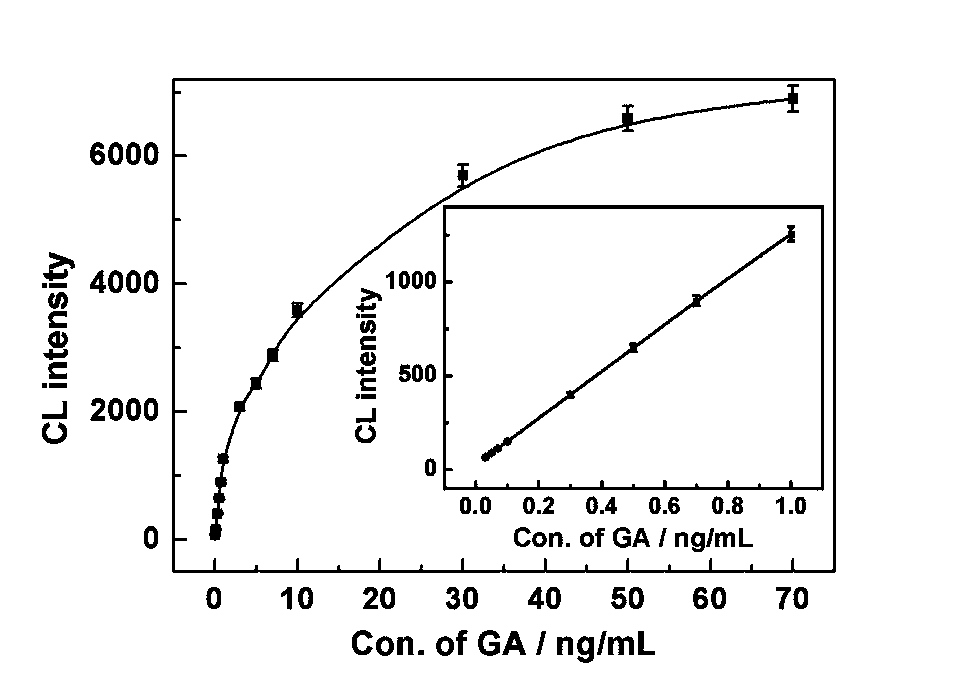
Method for determining gibberellin based on hybridization chain-reaction signal amplification technology
A technology of hybridization chain reaction and signal amplification, which is applied in the direction of chemical reaction of materials for analysis, biological testing, material inspection products, etc., and can solve the problems of cumbersome pretreatment methods and low sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
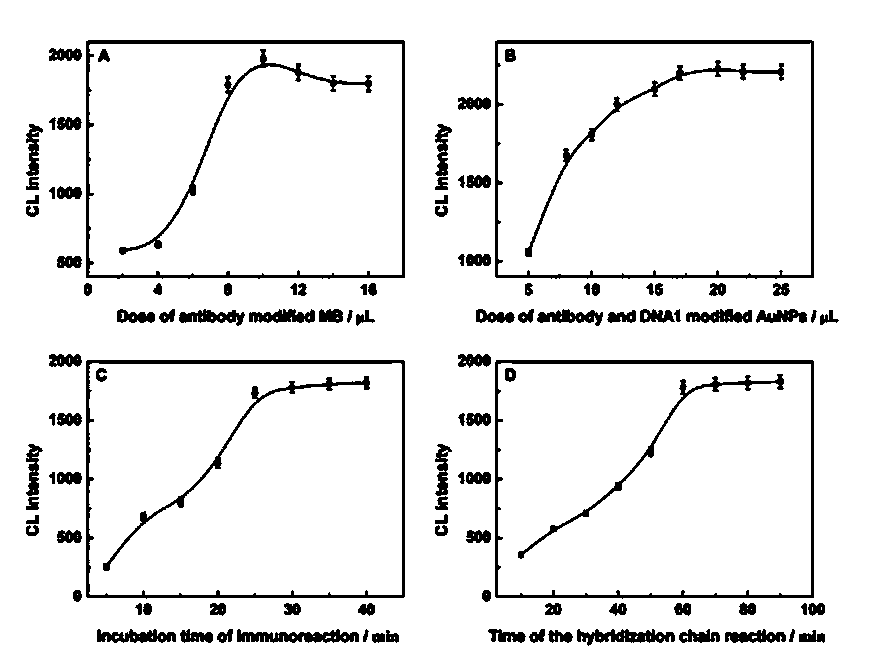
[0054] Example 1 Effect of the amount of antibody-modified magnetic beads on the intensity of chemiluminescence
[0055] According to the technical scheme, the test of the influence of the amount of antibody-modified magnetic beads on the intensity of chemiluminescence was carried out. The test results are as follows: figure 2 As shown in (A), the intensity of chemiluminescence increases gradually when the amount of antibody-modified magnetic beads increases from 2 μL to 10 μL. If the amount of antibody-modified magnetic beads is increased, the intensity of chemiluminescence decreases slowly, so 10 μL of antibody-modified magnetic beads is preferably used in the present invention.
Embodiment 2
[0056] Example 2 Effect of the amount of antibody and DNA1 modified colloidal gold on the intensity of chemiluminescence
[0057] The effect of the amount of antibody and DNA1-modified colloidal gold on the chemiluminescence intensity was tested. When the amount of antibody-modified magnetic beads was kept at 10 μL, when the amount of antibody and DNA1-modified colloidal gold was increased from 5 μL to 15 μL, the chemiluminescence intensity continued to increase . Continue to increase the amount of antibody and DNA1-modified colloidal gold, the chemiluminescence intensity gradually increases, and finally reaches the maximum at 20 μL, and then remains at this level, almost unchanged, the test results are as follows figure 2 (B). This is because the reaction was saturated when the amount of antibody and DNA1-modified colloidal gold was 20 μL. Therefore, 20 μL is the optimum amount of antibody and DNA1 modified colloidal gold. Therefore, the dosage of the antibody of the pres...
Embodiment 3
[0058] Example 3 Effect of reaction time between standard solution and antibody-labeled magnetic beads on chemiluminescent intensity
[0059] When the reaction between the standard solution and the antibody-labeled magnetic beads changed from 5 minutes to 30 minutes, the chemiluminescent intensity increased sharply, and then tended to be stable. The results are as follows: figure 2 (C) shown. Continuing to increase the reaction time does not cause a significant increase in the intensity of chemiluminescence. Therefore, the reaction time between the sample of the present invention and the antibody-labeled magnetic beads is preferably 30 minutes.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com