Controllable preparation method of Ag-manganese monoxide nanorods
A technology of manganese monoxide and nanorods, which is applied in chemical instruments and methods, metal/metal oxide/metal hydroxide catalysts, chemical/physical processes, etc., and can solve problems such as the coexistence of multivalent states
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
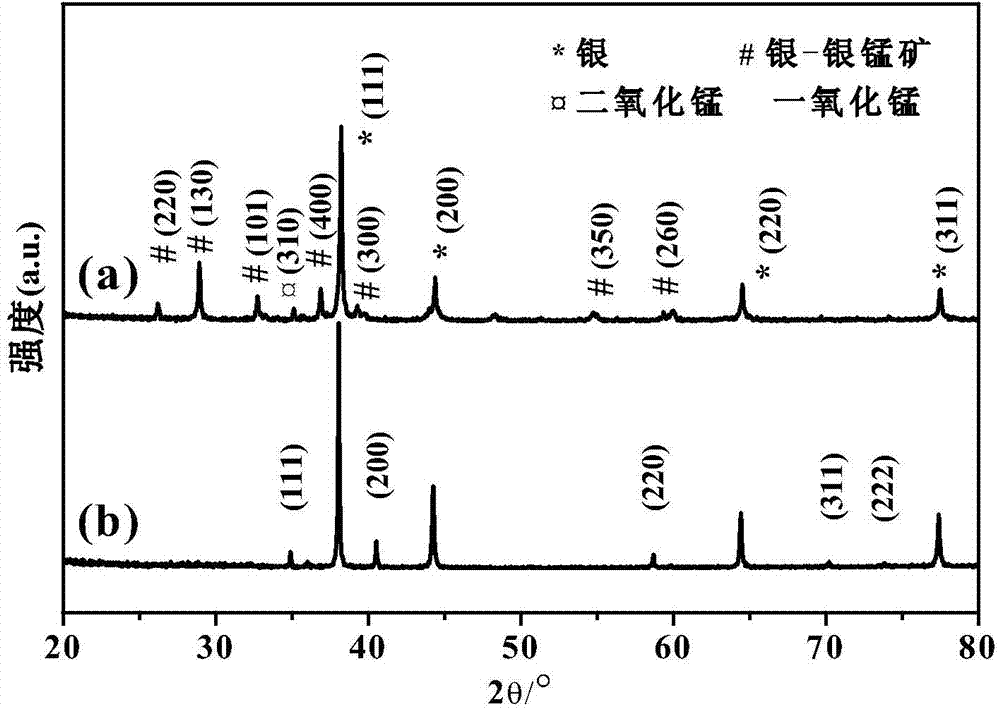
[0019] (1) Using silver nitrate and manganese (II) acetate as raw materials and deionized water as solvent, prepare 10 mL of 10 mM silver nitrate solution and 100 mL of 20 mM manganese acetate solution. 10mg of polyvinylpyrrolidone (PVP, K=30000) is a surfactant. (2) Add the silver nitrate solution dropwise to the manganese acetate aqueous solution. After the reaction, let it stand overnight, and then centrifuge and wash it several times to collect the precipitate. (3) Finally, the precipitate was dried at 80°C and ground, and the obtained powder was placed in a tube furnace under the protection of helium (the temperature rising rate was 20°C / min, from room temperature to 600 ℃), roasted for 5h to obtain. figure 1 It is the XRD spectrum of the prepared a sample and the product after roasting at 600°C.
Embodiment 2
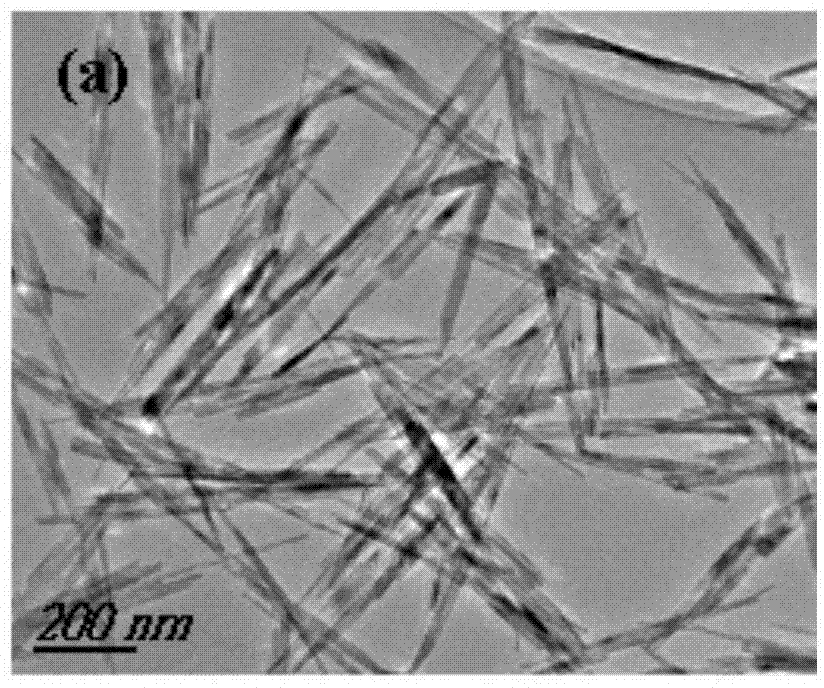
[0021] (1) Using silver acetate and manganese (II) nitrate as raw materials and deionized water as solvent, prepare 10 mL of 50 mM silver acetate solution and 100 mL of 200 mM manganese nitrate solution. 100mg of polyvinylpyrrolidone (PVP, K=30000) is a surfactant. (2) Add the silver acetate solution dropwise to the manganese nitrate aqueous solution. After the reaction, let it stand overnight, and then centrifuge and wash it several times to collect the precipitate. (3) Finally, the precipitate was dried at 80°C and ground, and the obtained powder was placed in a tube furnace under the protection of helium (the temperature rising rate was 1°C / min, from room temperature to 900°C) and roasted for 1h. figure 2 TEM spectra of the prepared samples.
Embodiment 3
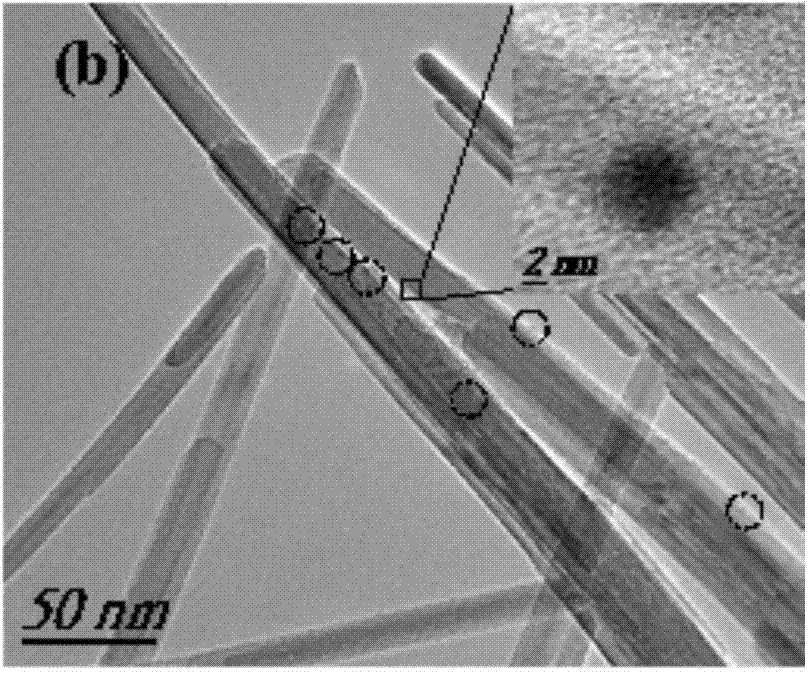
[0023] (1) Using silver nitrate and manganese (II) acetate as raw materials and deionized water as solvent, prepare 10mL of 10mM silver nitrate solution and 100mL of 200mM manganese acetate solution. 56mg of polyvinylpyrrolidone (PVP, K=30000) is a surfactant. (2) Add the silver nitrate solution dropwise to the manganese acetate aqueous solution. After the reaction, let it stand overnight, and then centrifuge and wash with acetone / water several times to collect the precipitate. (3) Finally, the precipitate was dried at 80°C and ground, and the obtained powder was placed in a tube furnace under the protection of helium (the temperature rising rate was 5°C / min, from room temperature to 800 ℃), roasted for 4h to obtain. image 3 TEM spectra of the prepared samples.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com