Oral recombinant yeast and application of target enteric canal DC (dentritic cell) presenting shRNA (short hairpin ribonucleic acid) mediated thereby
A technology of recombinant yeast and intestinal tract, applied in the fields of immunology and genetic engineering, can solve the problems of long time, high cost, complicated operation, etc., and achieve the effect of overcoming the long time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0029] The present invention will be further described in detail below in conjunction with specific embodiments, which are explanations of the present invention rather than limitations.
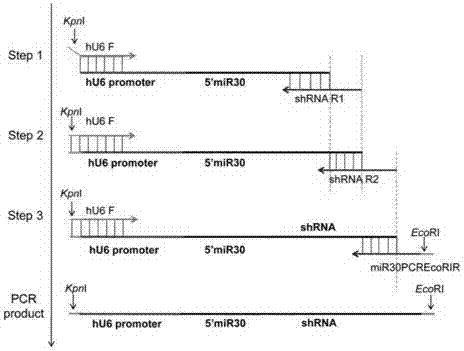
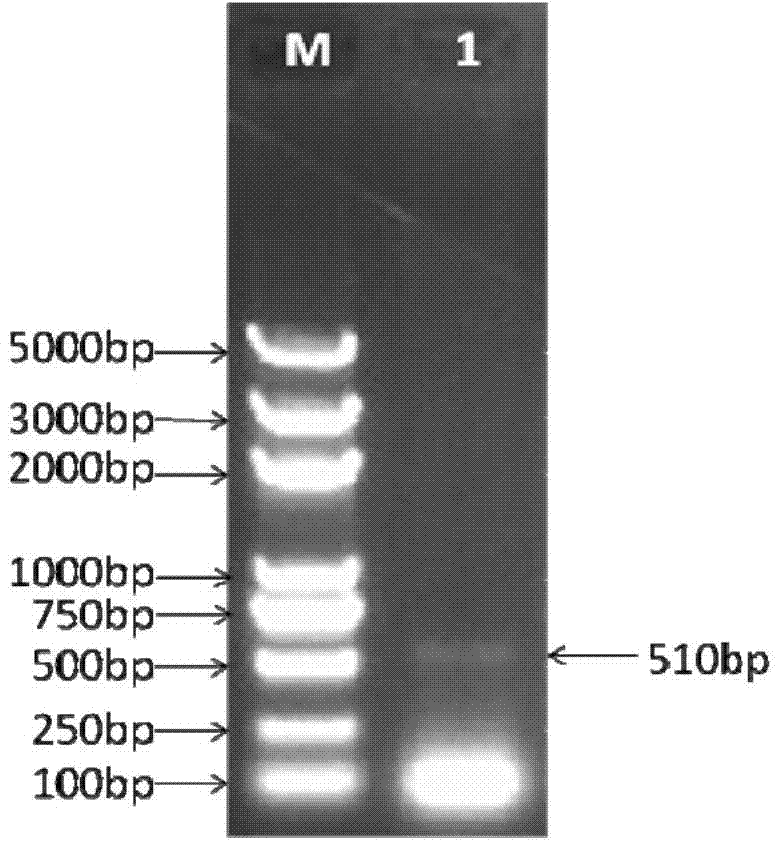
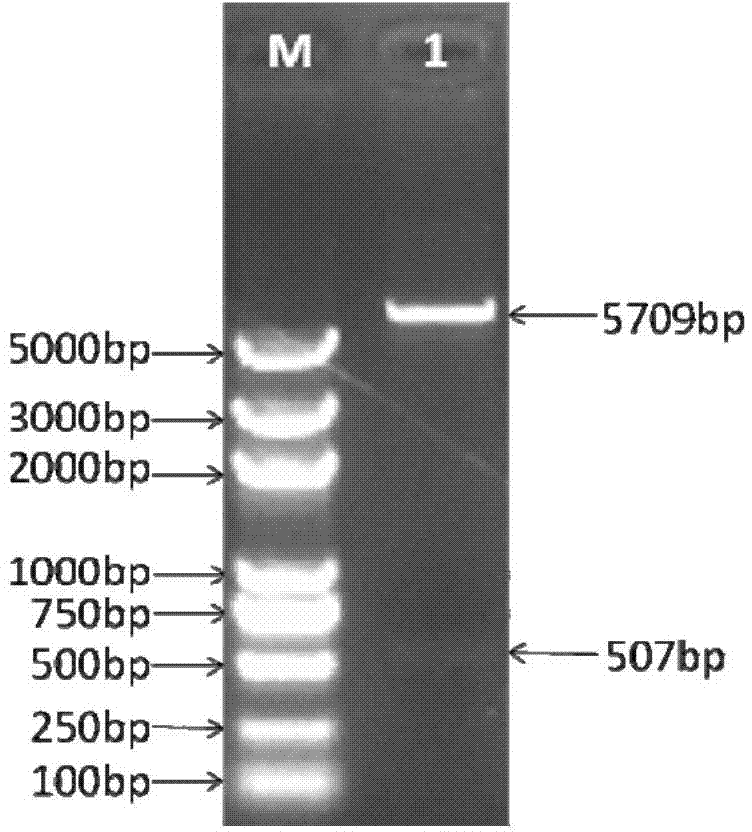
[0030] 1. Construction of JMB84-hU6 vector
[0031] 1. Using human genomic DNA as a template, use Clone ManagerV7 software to design primers, design primers hU6-F and hU6-R that can specifically amplify the hU6 promoter sequence, and introduce KpnI restriction sites into the upstream primer hU6-F , a SalI restriction site was introduced into the downstream primer hU6-R. The sequence design is shown in Table 1-1:
[0032] Table 1-1 PCR amplification primers of hU6 promoter gene
[0033] Primer name
Primer sequence
hU6-F
5′-TTG GGTACC CCCGAGTCCAACACCCGTGGG-3′
hU6-R
5′-CTA GTC GAC TAGTATATGTGCTGCCGAAGCG-3′
[0034] Note: The underlined part in the hU6-F primer sequence is the KpnI restriction site; the underlined part in the hU6-R primer sequenc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com