Methods for determining ligand binding to a target protein using a thermal shift assay
A target protein and protein technology, which is applied in the field of determining the binding of ligands to unpurified target proteins, can solve the problems of inability to use natural cells and tissues, affect the stability of GFP, and be unsatisfactory, and achieve the effect of easy multiplexing.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1- 4
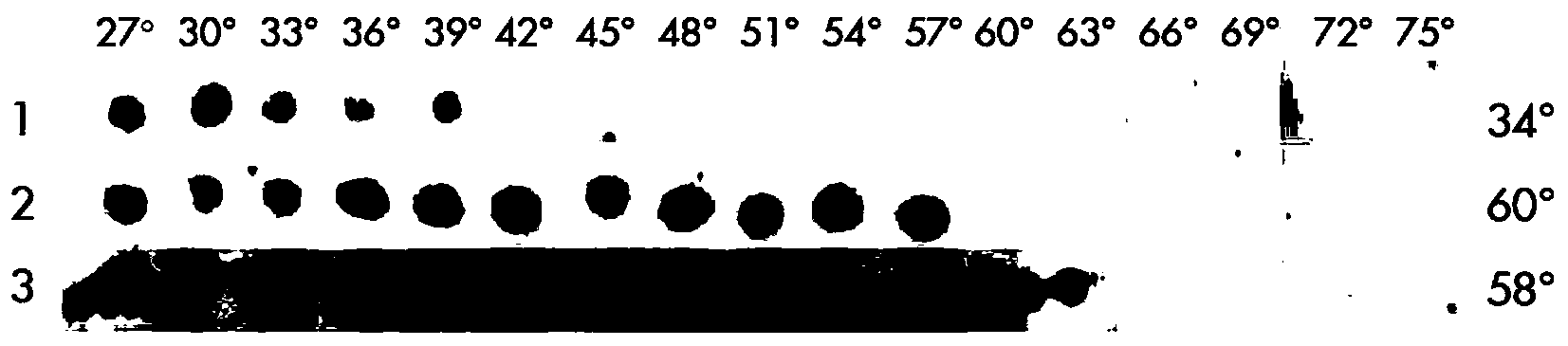
[0119] The mensuration of embodiment 1-four kinds of test protein melting temperature
[0120] To determine the melting temperature of each protein in cells, three human soluble protein expression constructs in expression vectors with an N-terminal His-tag were used. This is done by exposing the protein-containing cells to a set of increasing temperatures, and after each temperature step, the cells are spotted onto a "lysis / filter sandwich" submerged in lysis buffer. By using this lysis / filtration step, soluble proteins (up to the protein's specific melting temperature) can be detected as dark spots on the capture nitrocellulose membrane, whereas precipitated proteins (i.e., above their specific melting temperature) cannot passed through the filter and was therefore not detected.
[0121] Materials and methods
[0122]In 96-well deep-well plates (Porvair Plc., UK), by inoculating 1 ml containing 50 μg / ml kanamycin (Sigma-Aldrich Co., USA) and 35 μg / ml chloride with frozen E....
Embodiment 2
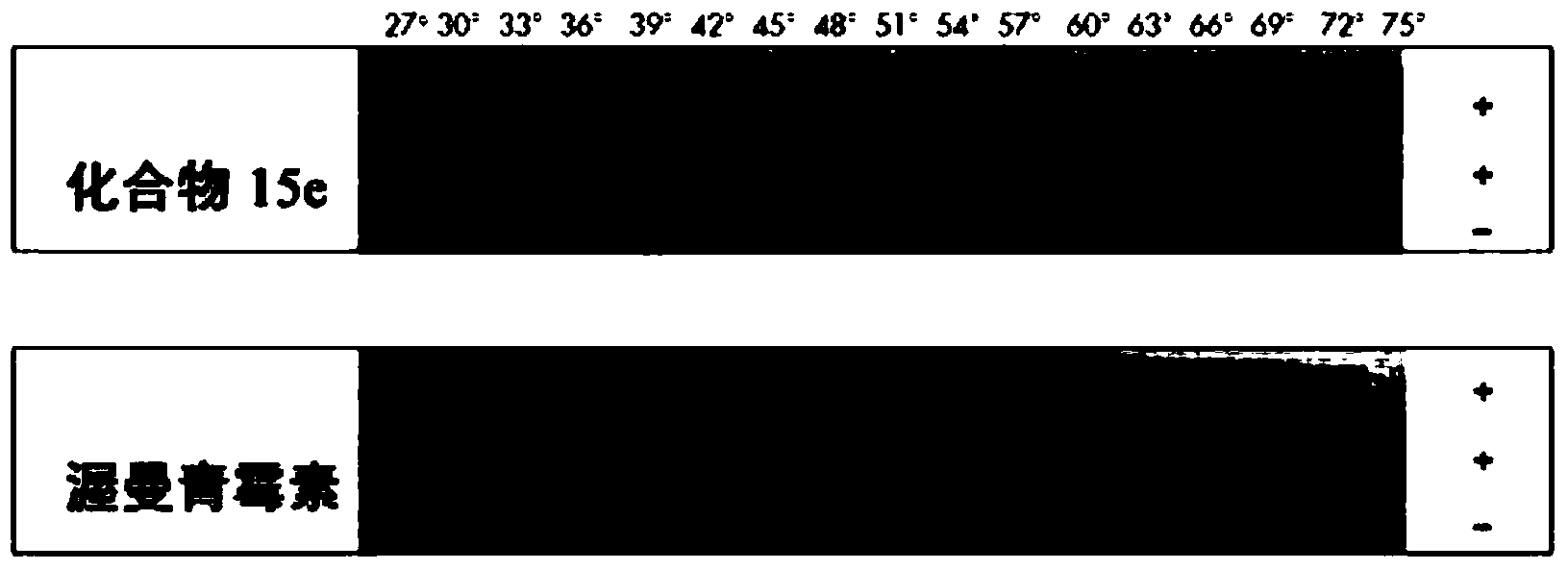
[0125] Example 2 - Detection of Increased Melting Temperature After Ligand Binding
[0126] Post-addition and binding of either of two PIK3C3-specific inhibitors (wortmannin and compound 15e) to study PIK3C3 constructs with expression constructs of human soluble PIK3C3 protein in expression vectors with N-terminal His-tag Possible increase in melting temperature. After treatment with or without one of the two inhibitors, cells expressing the PIK3C3 construct were exposed to a set of increasing temperatures, and after each temperature step, the protein-expressing cells were spotted submerged in lysis buffer on the "lysing / filtering sandwich". By using this lysis / filtration step on a "lysis / filtration sandwich", soluble proteins (up to the specific melting temperature of the construct) can be detected as dark spots on capture nitrocellulose membranes, whereas precipitated proteins (i.e., high due to its specific melting temperature) does not pass through the filter and therefo...
Embodiment 3
[0131] Example 3 - Determination of the melting temperatures of four test proteins in mammalian cell systems
[0132] To determine the melting temperatures of the four proteins, lysates were prepared from incubated mammalian cells and exposed to a set of increasing temperatures. After the temperature step, the precipitated protein is removed, leaving only the soluble protein to be detected (i.e., up to the specific melting temperature of the protein).
[0133] Materials and methods
[0134]Lysates were prepared from incubated human adenocarcinoma cells (A549). Cells were disrupted on ice in hypotonic buffer and homogenized. The suspension was freeze-thawed multiple times and after complete lysis all insoluble aggregates and cell debris were pelleted by centrifugation. The supernatant containing the visually clear cytoplasmic protein fraction was aliquoted into 8-strip PCR tubes and subjected to a set of increasing temperatures. After heating for three minutes, the samples ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com