Marbofloxacin-calcium chelate and synthetic method and application thereof
A technology of calcium chelate and marbofloxacin, which is applied in the direction of calcium organic compounds, drug combinations, antibacterial drugs, etc., can solve the problems of metal complexes or chelate synthesis and applications that have not been reported publicly, etc. Achieve the effect of improving immunity and promoting growth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
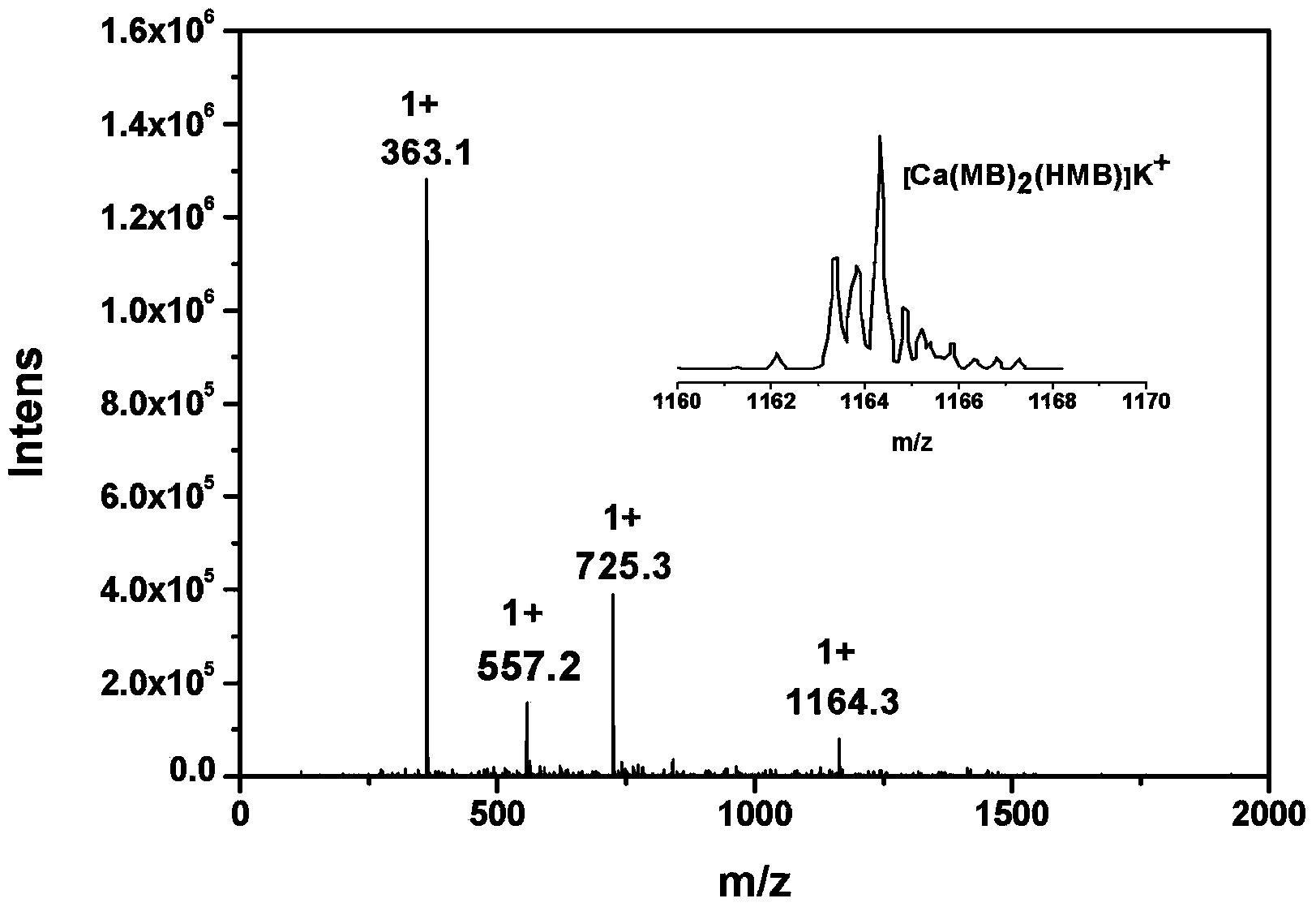
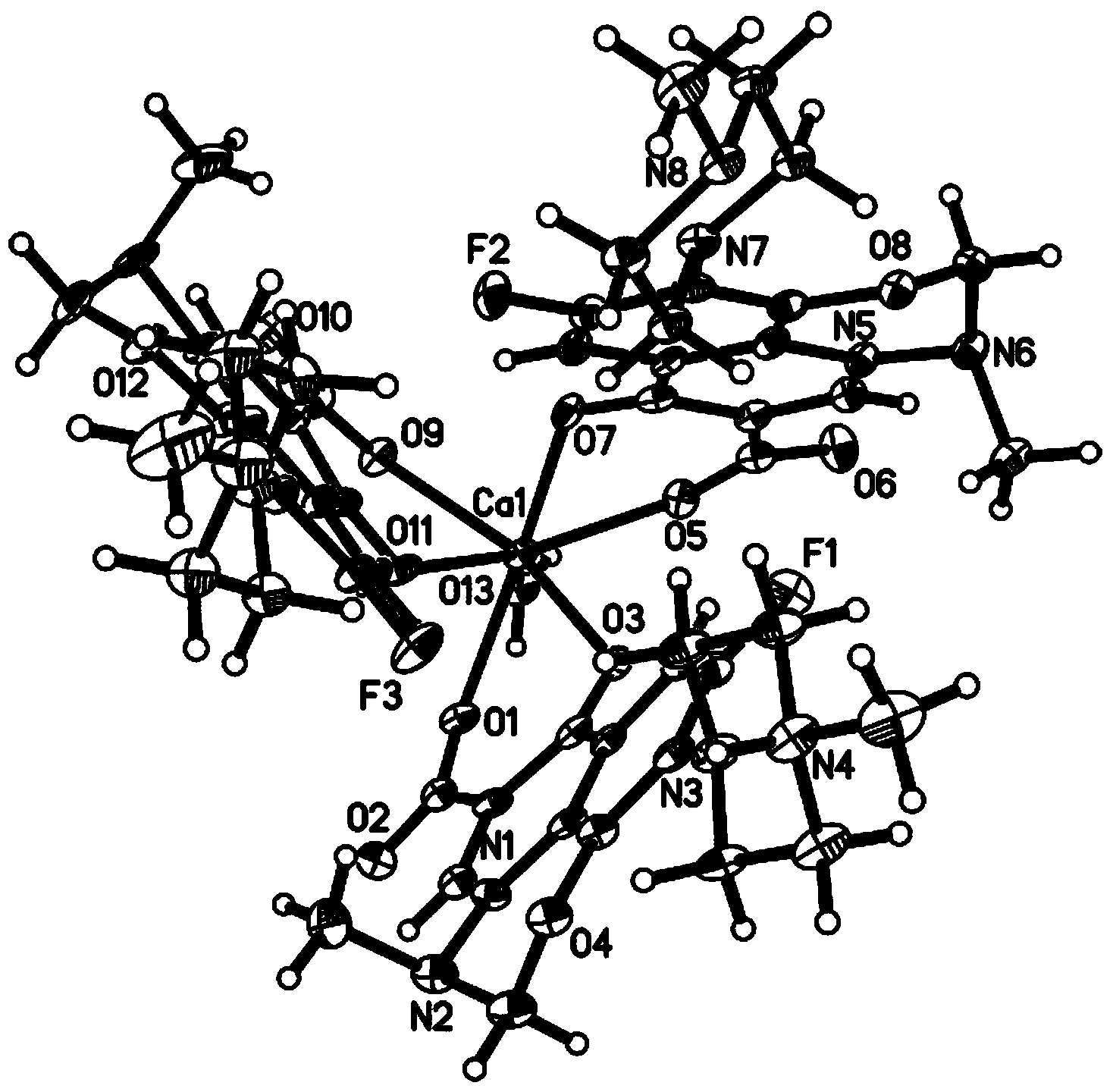
[0030] Take 3mmol of H-Marbo dissolved in 15mL of water to form a suspension; take 3mmol of triethylamine and add it dropwise to the H-Marbo suspension, stir and react at room temperature, and the solution gradually becomes clear. Add 1mmol of Ca(NO 3 ) 2 4H 2 O was dissolved in 5 mL of water, added dropwise to the above aqueous solution, stirred and reacted at room temperature for 12 hours, a large amount of precipitates formed. Filtration, the precipitate was washed with a small amount of cold water and ethanol in turn, and vacuum-dried at room temperature for 4 hours to obtain white microcrystals, which were determined to be the target chelate [Ca II (Marbo) 2 (Marbo-H + )(H 2 (0)] (yield: 75%).
Embodiment 2
[0032] Dissolve 3mmol of H-Marbo in 100mL of water to form a suspension; dissolve 3mmol of triethylamine in 10mL of water and add it to the suspension of H-Marbo, the solution gradually becomes clear. Add 1mmol of Ca(NO 3 ) 2 4H 2O was dissolved in 20 mL of water, added dropwise to the above aqueous solution, and reacted with stirring at 50°C for 5 hours. After the reaction, the aqueous solution was clear without precipitation. The reaction solution was evaporated and concentrated to about 20mL under reduced pressure to remove most of the water solvent, and then 100mL of ethanol was added to mix to precipitate the product, and a large amount of precipitate was precipitated after sufficient cooling. Filter, wash the precipitate with a small amount of cold water and ethanol in turn, and dry in vacuum at 40°C for 1 hour to obtain white microcrystals, which were determined to be the target chelate [Ca II (Marbo) 2 (Marbo-H + )(H 2 (0)] (yield: 80%).
Embodiment 3
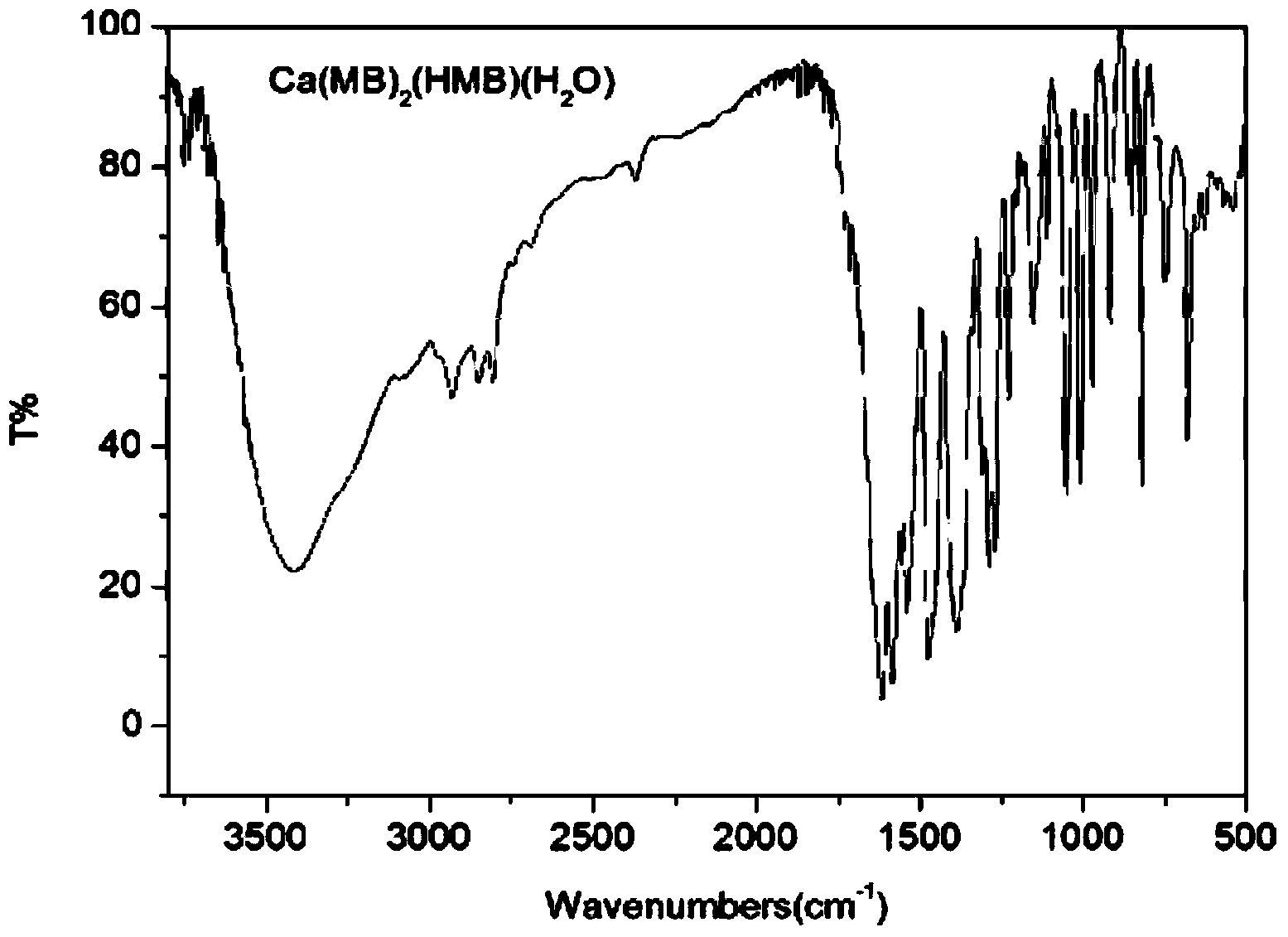
[0034] Take 3mmol of H-Marbo and dissolve it in 80mL of water / ethanol mixed solvent (the volume ratio of water and ethanol is 1:1) to form a suspension; take 3mmol of triethylamine and add it dropwise to the mixture of H-Marbo In suspension, the solution gradually became clear. Add 1mmol of Ca(NO 3 ) 2 4H 2 O was dissolved in 10 mL of water, added dropwise to the above aqueous solution, and reacted with stirring at 60°C for 3 hours. After the reaction, the aqueous solution was clear without precipitation. The reaction solution was evaporated and concentrated to about 20 mL under reduced pressure to remove most of the solvent, and then 100 mL of ethanol was added to mix to precipitate the product, and a large amount of precipitate was precipitated after sufficient cooling. Filter, wash the precipitate with a small amount of cold water and ethanol in turn, and dry it under normal pressure at 40°C for 3 hours to obtain white microcrystals. The structure was determined by infr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com