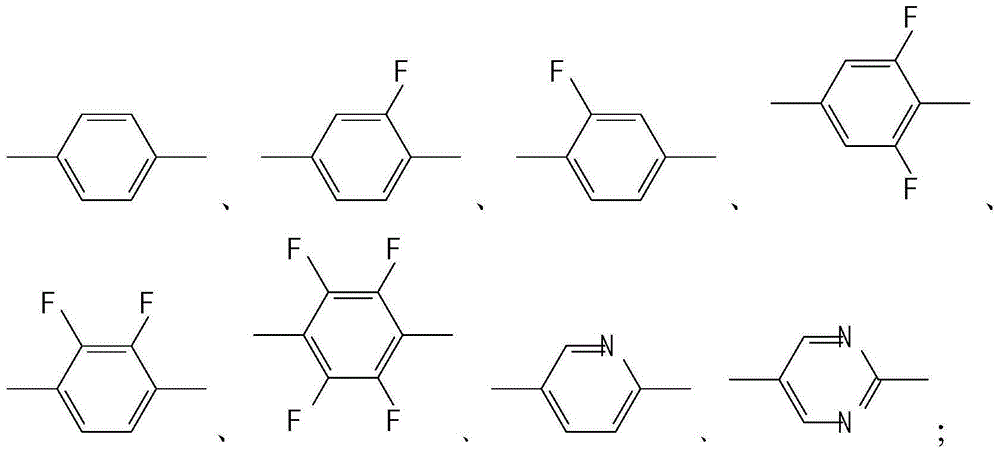
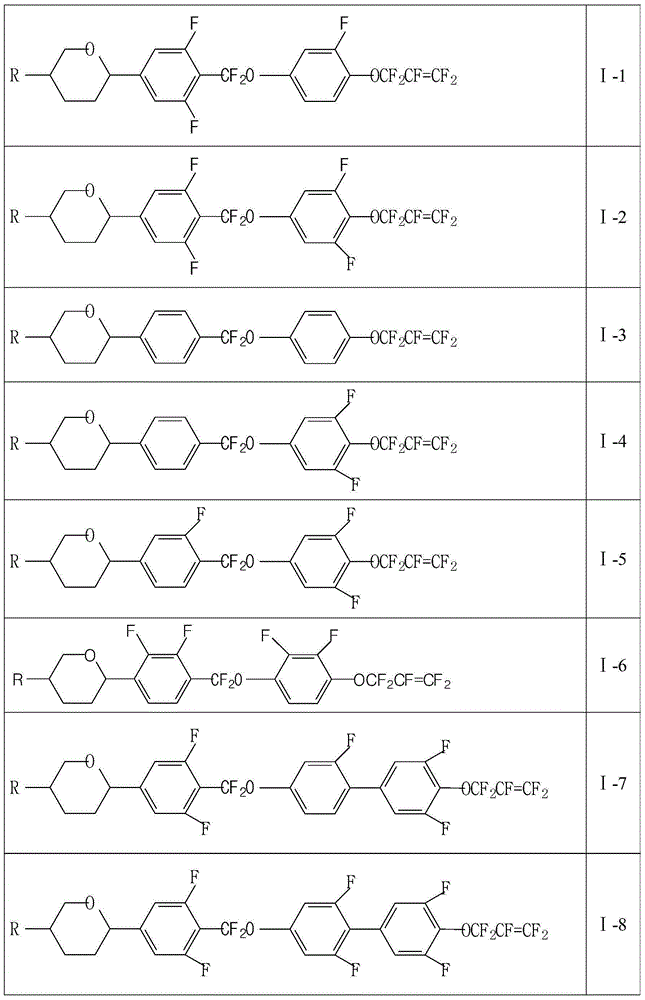
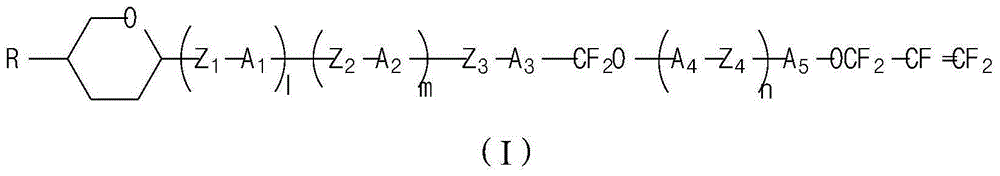A liquid crystal compound containing pentafluoropropene and pyran ring and its liquid crystal composition
A pentafluoropropene and compound technology, applied in liquid crystal materials, organic chemistry, chemical instruments and methods, etc., can solve the problems of small dielectric anisotropy, large rotational viscosity, slow response speed, etc., and achieve dielectric anisotropy. Large, fast response, small rotating sticky effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] BYLC-01
[0065] The synthetic route for the preparation of compound BYLC-01 is shown below:
[0066]
[0067] Synthesis of Compounds 1-2
[0068] Add 66.8g of magnesium chips (4.046mol) and 100ml of tetrahydrofuran into a 2L three-necked flask, heat up to reflux, add a small amount of solution consisting of 488g of 3,5-difluorobromobenzene (4.046mol) and 1000ml of tetrahydrofuran dropwise to initiate a reaction, under reflux The remaining solution of 3,5-difluorobromobenzene in tetrahydrofuran was added dropwise, the dropping was completed, and the reaction was carried out at 50 degrees for 0.5 hours to obtain Grignard reagent 1-1.
[0069] Add 287g (2.023mol) of propylpyranone and 750ml of tetrahydrofuran to a 5L three-necked flask, cool down to -30°C with liquid nitrogen, and add the prepared Grignard reagent 1-1 (4.046mol) dropwise under temperature control to -30°C. , the temperature was controlled at -30 °C and reacted for 1 hour, and then the temperature w...
Embodiment 2
[0087] BYLC-02
[0088]The synthetic route for the preparation of compound BYLC-02 is shown below:
[0089]
[0090] Synthesis of Compound 2-2
[0091] Add 66.8g of magnesium chips (4.335mol) and 100ml of tetrahydrofuran to a 2L three-necked flask, heat up to reflux, add a small amount of a solution consisting of 488g of 3,5-difluorobromobenzene (2.89mol) and 1000ml of tetrahydrofuran dropwise to initiate a reaction, under reflux The remaining solution of 3,5-difluorobromobenzene in tetrahydrofuran was added dropwise, the dropping was completed, and the reaction was carried out at 60 degrees for 5 hours to obtain Grignard reagent 2-1.
[0092] Add 287g (2.023mol) of propylpyranone and 750ml of tetrahydrofuran to a 5L three-necked flask, cool down to -35°C with liquid nitrogen, and add the prepared Grignard reagent 2-1 (2.89mol) dropwise at a temperature controlled to -35°C. , the temperature is controlled at -35 °C and reacted for 5 hours, and then the temperature is na...
Embodiment 3
[0110] BYLC-03
[0111] The synthetic route for the preparation of compound BYLC-03 is shown below:
[0112]
[0113] Synthesis of compound 3-2
[0114] Add 66.8g of magnesium chips (4.046mol) and 100ml of tetrahydrofuran into a 2L three-necked flask, heat up to reflux, add a small amount of solution consisting of 488g of 3,5-difluorobromobenzene (2.023mol) and 1000ml of tetrahydrofuran dropwise to initiate a reaction, and under reflux The remaining solution of 3,5-difluorobromobenzene in tetrahydrofuran was added dropwise, the dropping was completed, and the reaction was carried out at 65 degrees for 10 hours to obtain Grignard reagent 3-1.
[0115] Add 287g (2.023mol) of propylpyranone and 750ml of tetrahydrofuran into a 5L three-necked flask, cool down to -40°C with liquid nitrogen, and add the prepared Grignard reagent 3-1 (2.023mol) dropwise under temperature control to -40°C. , the temperature is controlled at -40 °C and reacted for 10 hours, and then the temperat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com