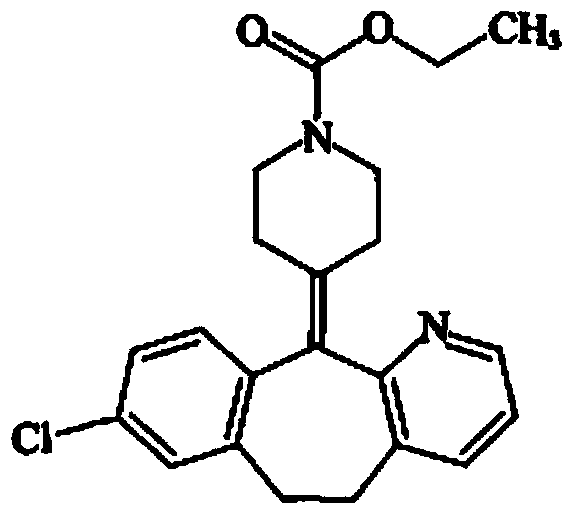
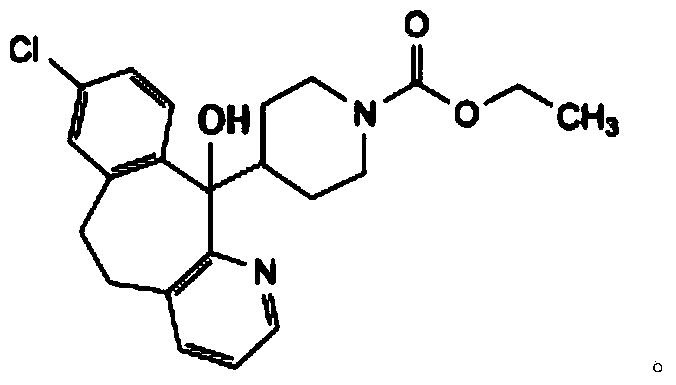
Liquid loratadine composition
A technology of loratadine and composition, which is applied in the directions of drug combination, drug delivery, medical preparations of inactive ingredients, etc., can solve problems such as cumbersome
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment A1
[0112] Embodiment A1: Preparation of the composition of the present invention
[0113] Prescription (prepared in 1000 servings, the prescription for each serving is as follows):
[0114]
[0115] Preparation method: take loratadine, add about 90% of the formula oil, stir to dissolve, add oil to the full amount, and stir evenly to obtain the composition of the present invention, which is a transparent liquid without sediment.
[0116] Half of the liquid medicine of the present embodiment composition prepared above is packed in a glass bottle (also can be a plastic bottle such as a high-density polyethylene bottle), and the other half is prepared into a soft capsule preparation as follows:
[0117] Capsule skin formula: 40% gelatin, 18% glycerin, 7% sorbitol, 35% water.
[0118] By the following steps, the above-mentioned liquid composition of the present invention is wrapped in prepared capsule skins, and each capsule contains 10 mg of active ingredient.
[0119] Take gel...
Embodiment A2
[0120] Embodiment A2: Preparation of the composition of the present invention
[0121] Prescription (prepared in 1000 servings, the prescription for each serving is as follows):
[0122]
[0123] Preparation method: Prepare according to the method of Example A1 to obtain the composition of the present invention. Further, a part of the composition is packaged in a glass bottle, and the other part is prepared into a soft capsule preparation.
Embodiment A3
[0124] Embodiment A3: Preparation of the composition of the present invention
[0125] Prescription (prepared in 1000 servings, the prescription for each serving is as follows):
[0126]
[0127] Preparation method: Prepare according to the method of Example A1 to obtain the composition of the present invention. Further, a part of the composition is packaged in a glass bottle, and the other part is prepared into a soft capsule preparation.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com