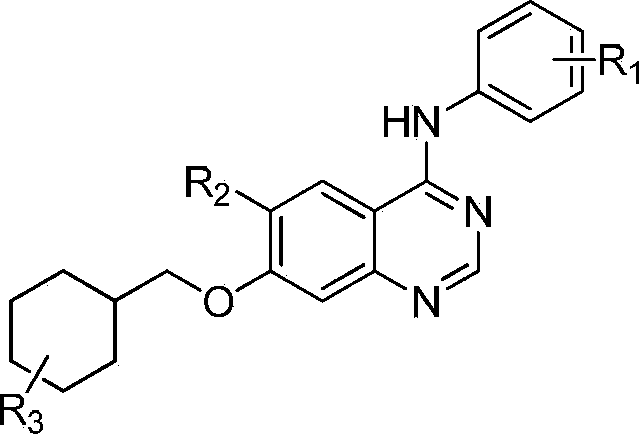
7-substituted cyclohexyl quinazoline derivatives and preparing method and uses thereof
A technology of quinazoline and cyclohexyl, applied in the preparation of antitumor drugs, 7-substituted cyclohexylquinazoline derivatives and its preparation field, can solve the problems of cardiac toxicity and side effects, prolonged cardiac QT time, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
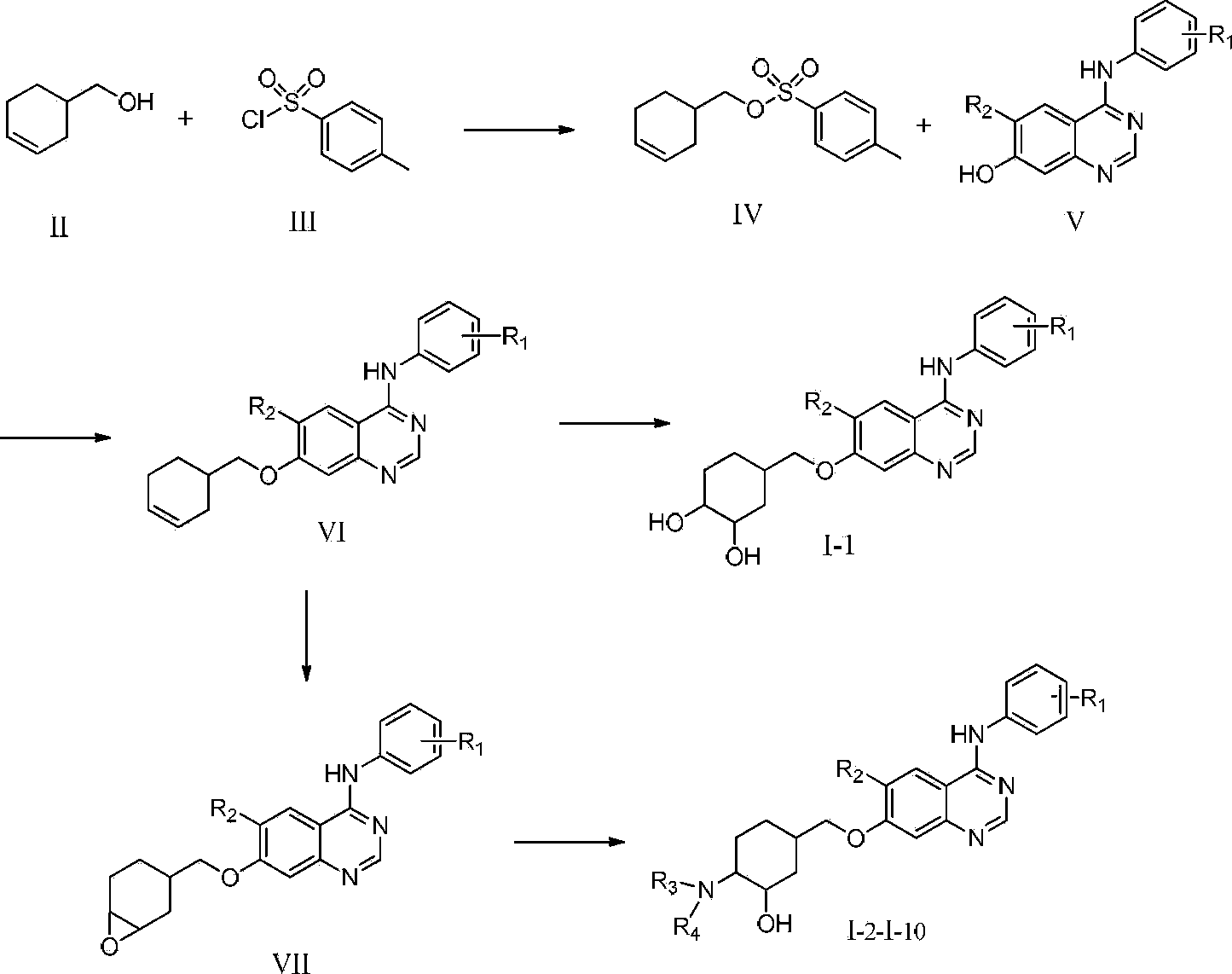
[0049] The preparation of embodiment 1 cyclohexenyl p-toluenesulfonate (IV)
[0050] In a 250mL three-necked flask, add 15g of 4-cyclohexenemethanol (13.4mmol), 90ml of pyridine, stir at room temperature, add 61.4g (32.2mmol) of p-toluenesulfonyl chloride, stir at room temperature for 5 hours, the reaction is complete, add 200ml In water, use concentrated hydrochloric acid to adjust the pH value to 7, wash twice with water, extract with ethyl acetate, dry over anhydrous magnesium sulfate, filter, and concentrate under reduced pressure until exhausted to obtain a yellow oil, which is directly carried out to the next reaction.
Embodiment 24
[0051] Example 2 Preparation of 4-(2-fluoro-4-bromophenyl)-6-methoxy-7-(3-cyclohexenyl)methoxy)quinazoline (VI-1)
[0052] Add IV (1g, 3.76mmol), 4-((4-bromo-2-fluorophenyl)amino)-6-methoxy-7-hydroxyquinazoline (1g, 2.89mmol) to a 50ml round bottom flask , anhydrous potassium carbonate (0.8g, 5.78mmol), DMF (20ml), reacted at 80°C for 2 hours, cooled to room temperature, added 25ml of water under stirring, and precipitated a large amount of green solid. After the precipitation was complete, filtered to obtain a green solid. A white solid (0.68 g, yield 52.3%) was obtained by rapid preparative liquid chromatography column separation.
[0053] 1 H NMR (400MHz, DMSO-d 6 ):δ1.39(m,1H),1.88(m,2H),2.07(m,3H),2.49(d,1H),3.94(s,3H),4.03(m,2H),5.70(d, 2H),7.19(s,1H),7.45(d,1H),7.52(t,1H),7.64(d,1H),7.78(s,1H),8.34(s,1H),9.51(s,1H ). ESI-MS: m / z 457[M+H] + .
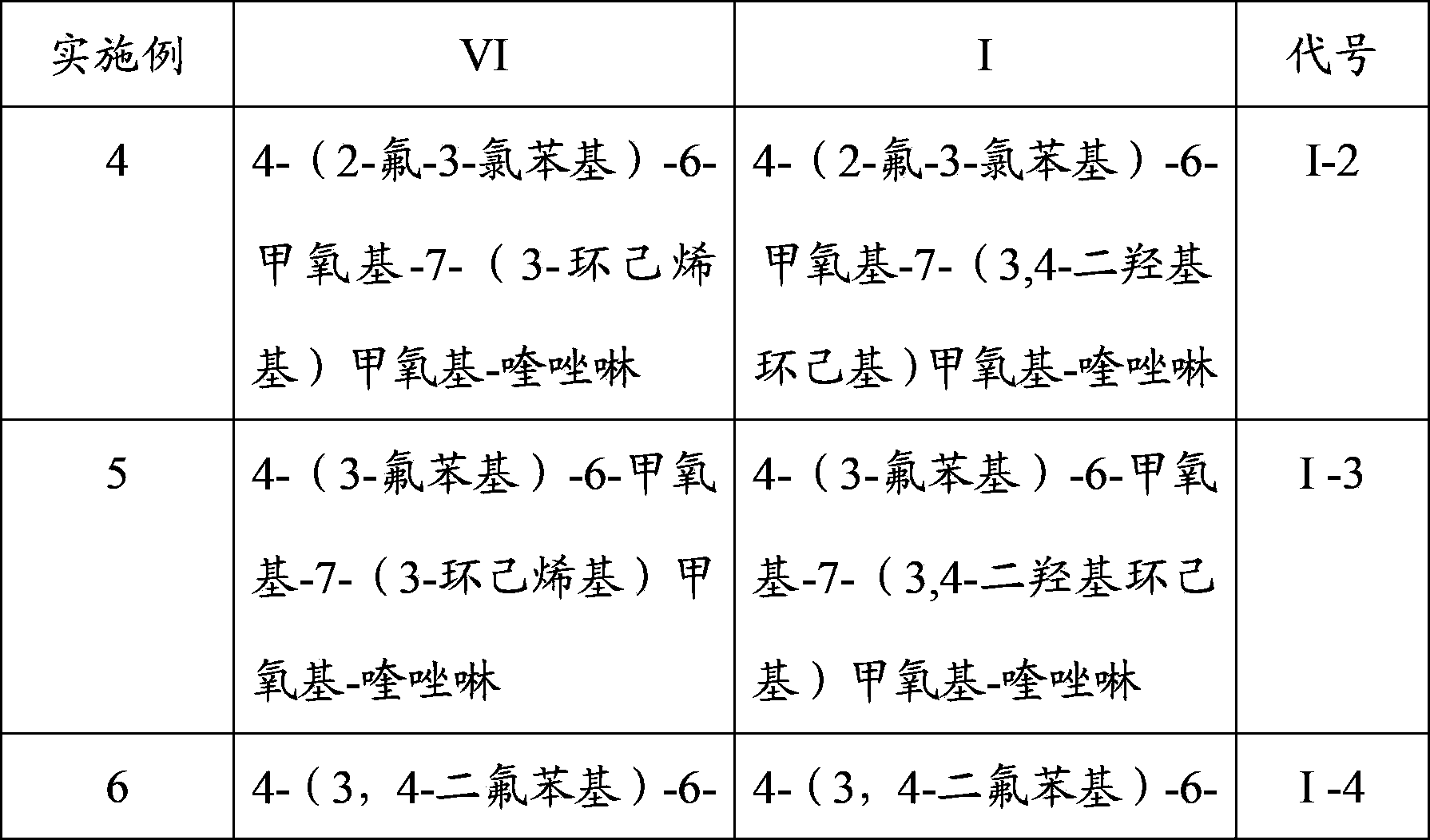
[0054] Using the same method to prepare 4-(2-fluoro-3-chlorophenyl)-6-methoxy-7-(3-cyclohexenyl)methoxy-quinazoline (VI-2...
Embodiment 34
[0055] Example 34-(2-fluoro-4-bromophenyl)-6-methoxy-7-(3,4-dihydroxy-cyclohexyl)methoxy)quinazoline (I-1)
[0056] Take 1g (2.19mmol) of VI-1, put it into a 50ml round bottom flask, add 10ml of THF, stir at room temperature, and dissolve it; take 0.52g (3.28mmol) of KMnO 4 , 20ml of distilled water, add an appropriate amount of NaOH to make the pH of the aqueous solution 7-8, and then start to add KMnO dropwise to the round bottom flask 4 The aqueous solution was added dropwise in 20 minutes, and the color of the reaction solution gradually changed from light yellow clear to brownish yellow turbid liquid. The reaction was continued at room temperature for 2 hours, followed by TLC until the reaction of the raw materials was completed, and the insoluble brown-yellow solid MnO was filtered off. 2 , concentrated the filtrate, and the remaining mixed solution was extracted 3 times with ethyl acetate 15ml, the organic layers were combined, anhydrous MgSO 4 Dry, filter, and concen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com