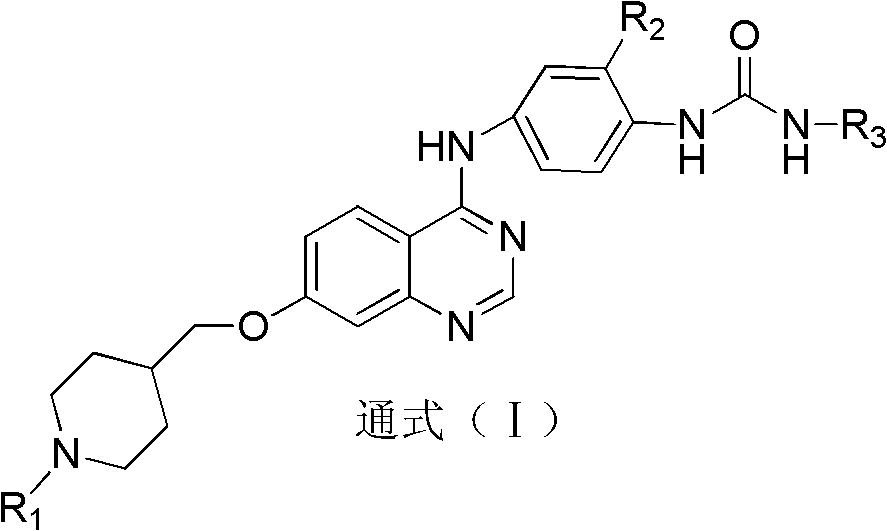
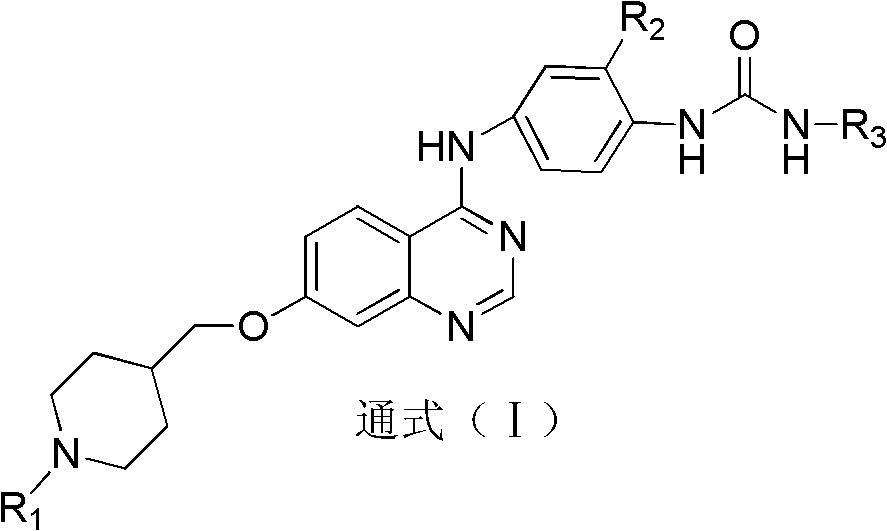
Quinazolinyl aryl urea derivatives and preparation method and application thereof
A kind of quinazoline aryl urea, quinazoline technology, applied in the field of drug synthesis, can solve the problem of aggravating side effects and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1 Preparation of 1-isocyanate-4-nitrobenzene
[0026] Add 5.92 g (20 mmol) of triphosgene and 20 mL of dichloromethane into a 250 mL three-necked flask, and stir at room temperature to dissolve it. 1.38g (10mmol) of p-nitroaniline was dissolved in 100mL of dichloromethane and dropped into the reaction solution. After the dropwise addition, slowly add triethylamine (10-15mL) solution diluted with 30mL of dichloromethane to the reaction solution until alkaline, continue the reaction for 1-1.5h after the dropwise addition, and proceed to the next reaction directly.
[0027] 2-Fluoro-1-isocyanate-4-nitrobenzene and 2-chloro-1-isocyanate-4-nitrobenzene were prepared using the same method.
Embodiment 2
[0028] Example 2 Preparation of 1-(3,4-difluorophenyl)-3-(4-nitrophenyl)urea
[0029] Add 1.29 g (10 mmol) of 3,4-difluoroaniline into a 500 mL three-necked flask, stir to dissolve in 20 mL of dichloromethane, and heat to reflux. Add the step reaction solution dropwise. After the dropwise addition, the reaction was continued for about 0.5h, and the reaction was stopped. After the reaction solution was evaporated to dryness, it was treated with 30 mL of acetone and 100 mL of water to obtain 1.7 g of a large amount of yellow solid, with a yield of 58.0%, m.p.78-79°C; 1 H NMR (400MHz, DMSO): δ7.15-7.17(m, 1H, ArH), 7.33-7.40(m, 1H, ArH), 7.63-7.73(m, 3H, ArH), 8.18-8.22(m, 2H , ArH), 9.11(s, 1H, NH), 9.48(s, 1H, NH), ESI-MS: m / z 294 [M+H] + .
[0030] Use the same method to prepare 1-(3-fluorophenyl)-3-(4-nitrophenyl)urea, 1-phenyl-3-(4-nitrophenyl)urea, 1-cyclopropyl- 3-(4-nitrophenyl)urea, 1-cyclohexyl-3-(4-nitrophenyl)urea, 1-(5-methyl-1,3,4-oxadiazol-2-yl )-3-(4-nitroph...
Embodiment 3
[0031] Example 3 Preparation of 1-(4-aminophenyl)-3-(3,4-difluorophenyl)urea (V-1)
[0032] Add 1-(3,4-difluorophenyl)-3-(4-nitrophenyl)urea 2.9g (10mmol), 150mL methanol and palladium carbon 0.4g (4mmol) into a 250mL round bottom flask, and continue to pass Enter H 2 React for 1-2 hours. After the reaction is complete, the palladium carbon is filtered out, and the mother liquor is evaporated to dryness to obtain the product, 2.5 g, with a yield of 86.2%. m.p.83-85℃; 1 H NMR (400MHz, DMSO): δ4.78(s, 2H, NH 2 ), 7.15-7.17(m, 1H, ArH), 7.33-7.40(m, 1H, ArH), 7.63-7.73(m, 3H, ArH), 8.18-8.22(m, 2H, ArH), 9.11(s, 1H, NH), 9.48 (s, 1H, NH), ESI-MS: m / z 264 [M+H] + .
[0033] Using the same method to prepare 1-(4-aminophenyl)-3-(3-fluorophenyl)urea (Ⅴ-2), 1-(4-aminophenyl)-3-phenylurea (Ⅴ -3), 1-(4-aminophenyl)-3-cyclopropylurea (Ⅴ-4), 1-(4-aminophenyl)-3-cyclohexylurea (Ⅴ-5), 1 -(4-aminophenyl)-3-(5-methyl-1,3,4-oxadiazol-2-yl)urea (Ⅴ-6), 1-(4-aminophenyl)- 3-(4-Chloro-3-(t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com