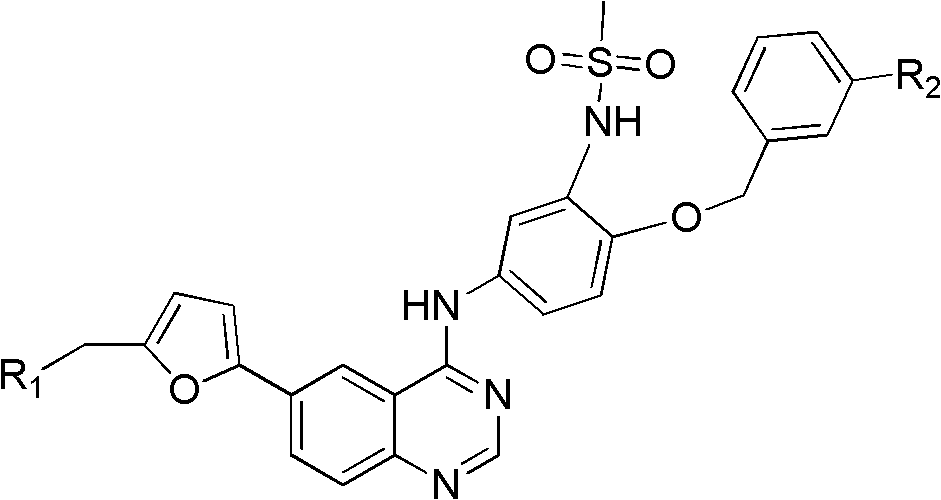
4-subsittution m sulfonylurea amide aniline-quinazoline derivate and preparation method and application thereof
A technology of quinazoline and derivatives, applied in the preparation of the 4-substituted m-methanesulfonamide anilino-quinazoline derivatives, the application field of anti-tumor drugs, can solve the problem of increasing the difficulty of curing and recurrence. probability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
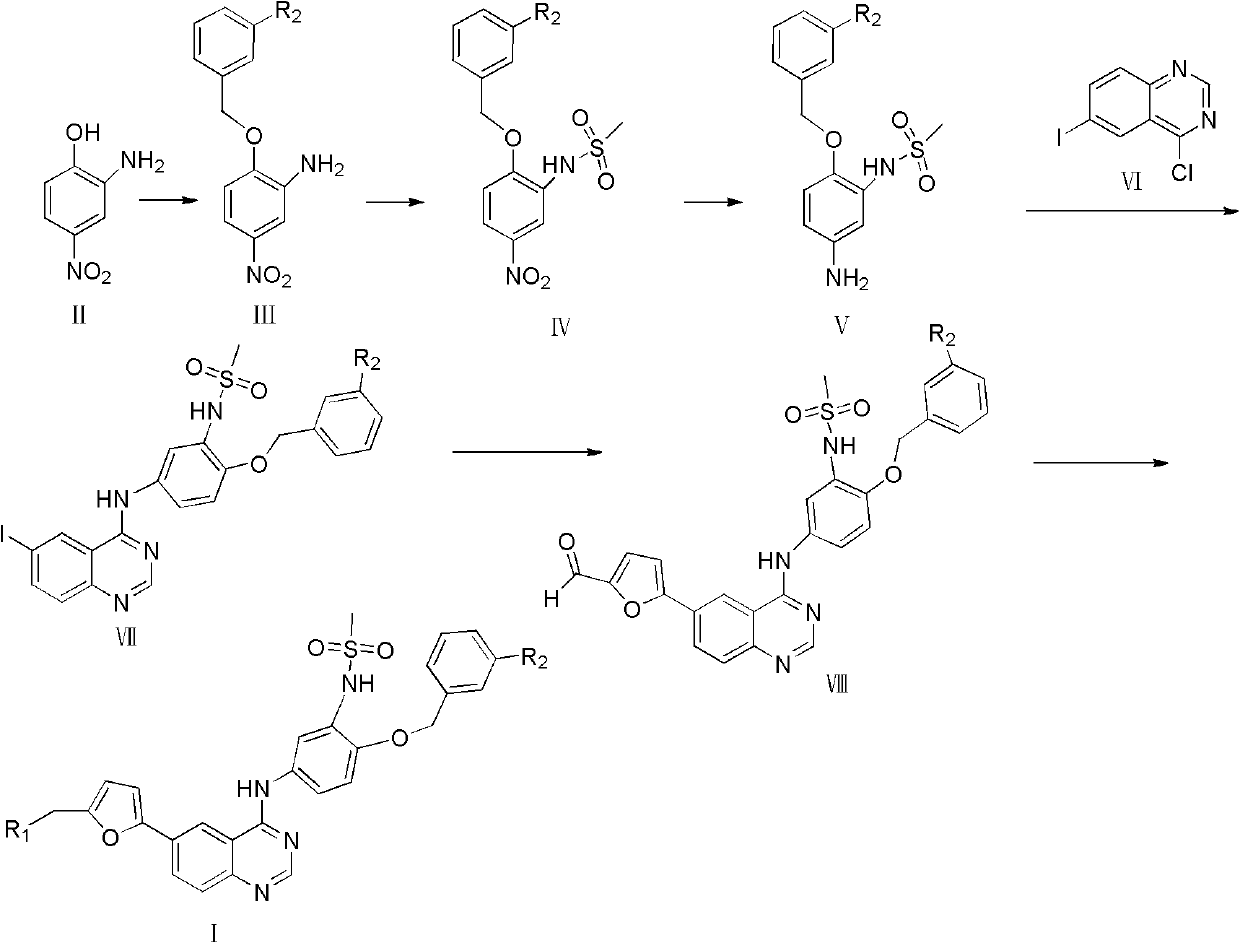
Embodiment 1
[0025] Example 1 Preparation of 2-benzyloxy-5-nitroaniline
[0026] Put 4g (25.97mmol) of 2-amino-4-nitrophenol, 4.9g (28.65mmol) of benzyl bromide, and 7.18g (52.02mmol) of potassium carbonate in a 100ml round bottom flask, add 30ml of acetone, and reflux at 60°C for 1.5 h, recrystallized from ethyl acetate to give 2-benzyloxy-5-nitroaniline (3.8g g, 58.8%) as a yellow solid, m.p.98-99°C; 1 H NMR (400MHz, DMSO): δ5.23(s, 2H, CH 2 O), 6.412 (s, 2H, NH 2 ), 6.6756.697 (d, 1H, ArH), 7.324-7.675 (m, 7H, ArH), ESI-MS: m / z 245 [M+H] + .
Embodiment 2
[0027] Example 2 Preparation of 1-(N-methylsulfonyl)-2-benzyloxy-5-nitroaniline
[0028] Put 1 g (4.10 mmol) of 2-benzyloxy-5-nitroaniline in a 100 ml round bottom flask, add 16 ml of DMF, and stir well. The system was placed in an ice bath, and 0.4 g of sodium hydrogen (16.67 mmol) was added in batches. Stir at room temperature for 30 min under nitrogen protection. Measure 1.42 g (12.40 mmol) of methanesulfonyl chloride, and slowly add it into the system. Stir overnight at room temperature. 30ml of water was added to the system, and a large amount of precipitation occurred. Filter and wash with water to obtain light yellow solid powder. It was dissolved in DMF, and the pH was adjusted to 9-10 with 3N aqueous sodium hydroxide solution. The reaction was carried out at 90° C. for 6 h, and then naturally cooled to room temperature. Adjust the pH to 1-2 with 5N hydrochloric acid, a large amount of precipitation appeared in the system, filtered and washed with water to obtain ...
Embodiment 3
[0029] Example 3 Preparation of N-(5-amino-2-(benzyloxy)phenyl)methanesulfonamide
[0030] 0.2 g (0.62 mmol) of 1-(N-methylsulfonyl)-2-benzyloxy-5-nitroaniline, FeCl 3 6H 2 O 0.67g (2.48mmol) was placed in a 50ml round bottom flask, and then 7ml of DMF and H 2 O (6:1), stirred at room temperature for 30 min. Weigh 0.4g Zn powder (6.13mmol), slowly add to the system, stir at room temperature for 3h, stop the reaction. Filter the reaction solution, take the filtrate, dilute it with 1-2 times the amount of water, then extract it with ethyl acetate, add HCl / ethyl acetate, a large amount of precipitation occurs, and filter it. Dissolve it in water, adjust the pH to alkaline, extract with ethyl acetate, and evaporate to dryness under reduced pressure to obtain N-(5-amino-2-(benzyloxy)phenyl)methanesulfonamide (0.16g, 92.3% ), m.p.103-104°C; 1 H NMR (400MHz DMSO): δ2.734(s, 3H, CH 3 ), 5.024 (s, 2H, NH 2 ), 5.154 (s, 2H, CH 2O), 6.094-7.524 (m, 8H, ArH), 8.46 (s, 1H, NH), ESI...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com