A kind of non-invasive helicobacter pylori gene detection kit and its preparation and detection method
A technology for Helicobacter pylori and gene detection, which is applied in biochemical equipment and methods, microbial determination/inspection, DNA/RNA fragments, etc., can solve the problems of human and material resources and detection time without cost saving, and achieve economical operation. The process and operation time, preparation method and operation steps are simple, and the specificity is good.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Design and specificity test of embodiment 1 primer
[0052] Using the 16sRNA of Helicobacter pylori as the target sequence, the primers were designed as follows:
[0053] Primer is 5'-TTGGGATAGCCATTGGAAACG-3'
[0054] 3'-GTTCCGATACTGCCCATAGGC-5';
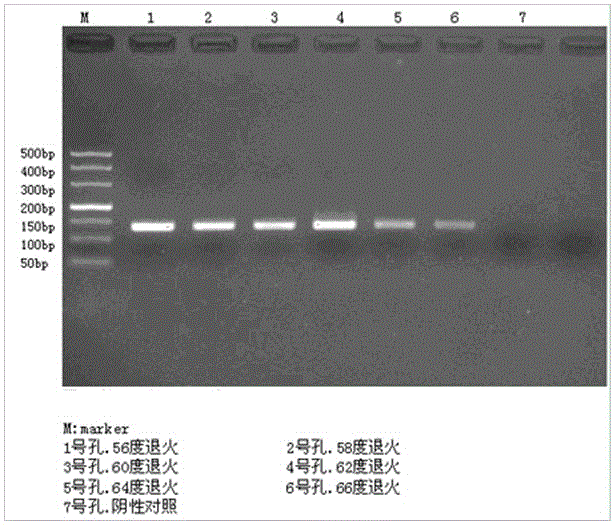
[0055] Using the 16sRNA of the strain---Helicobacter pylori Sydney strain Helicobacter pyloriSS1 (referred to as Hp) (purchased from Hebei Medical University, from the Institute of Epidemiology, Chinese Center for Disease Control and Prevention) as the target sequence, the primers obtained were located in the coding region 5 In the 300-400bp region at the 'end, the amplification product is between 100-200bp, the bases of the primers are randomly distributed and uniform, the primers themselves cannot form dimers, and the length of the primers is between 17-25 bases. The difference cannot be 3 bases, the G+C content is 45-55%, the primer is not in the area rich in AT and GC, there is no T / C, A / G continuous structure (2-3), an...
Embodiment 2
[0058] The amplification reaction of embodiment 2 primers
[0059] The above primer 5'-TTGGGATAGCCATTGGAAACG-3'
[0060] The PCR amplification reaction of 3'-GTTCCGATACTGCCCATAGGC-5' is:
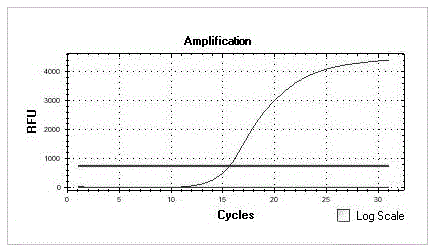
[0061] Melt the primer at 90-98°C for 8-10s; then cool down to 56-66°C for annealing and extension for 25-30s;
[0062] Two-step cycle 30cycle. The existing process is to lower the temperature to about 55°C for annealing treatment for a period of time, and then raise the temperature to about 72°C for extension, but the annealing extension of the present invention is a step, because the annealing extension adopted in the present invention is carried out under the same temperature condition Therefore, it is not necessary to cool the smelted product twice, but directly cool the product to the temperature required for extension to obtain the primer amplified fragment. Compared with the PCR amplification reaction of the existing primer, one cooling step is omitted , which saves the operation p...
Embodiment 3
[0065] Example 3 A kind of non-invasive Helicobacter pylori gene detection kit
[0066] A kit for non-invasive nucleic acid detection of Helicobacter pylori including R1 reagent: primer 5'-TTGGGATAGCCATTGGAAACG-3'
[0067] 3'-GTTCCGATACTGCCCATAGGC-5',
[0068] R2 reagent: SYBRGreenTaqMIX (CW0078, CWBio), other PCRTaq enzyme premixes are also available; R3 reagent: sterile distilled water (201305, self-made).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com