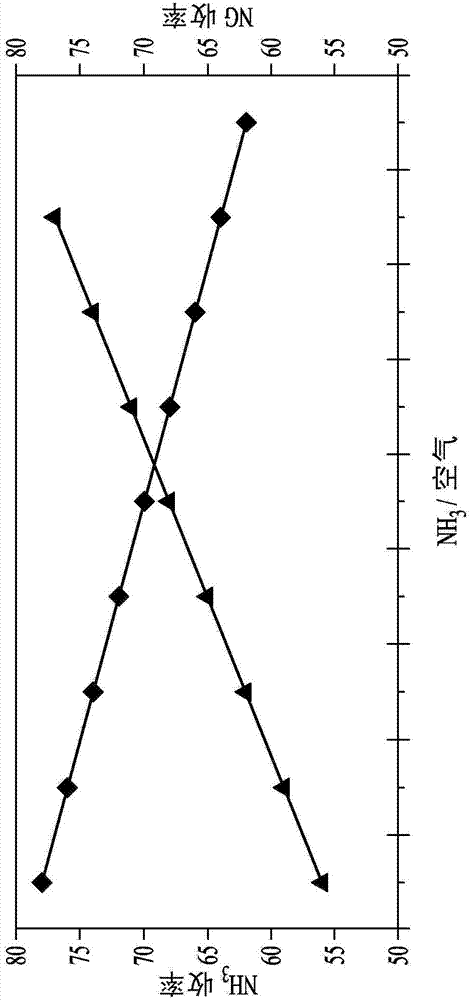
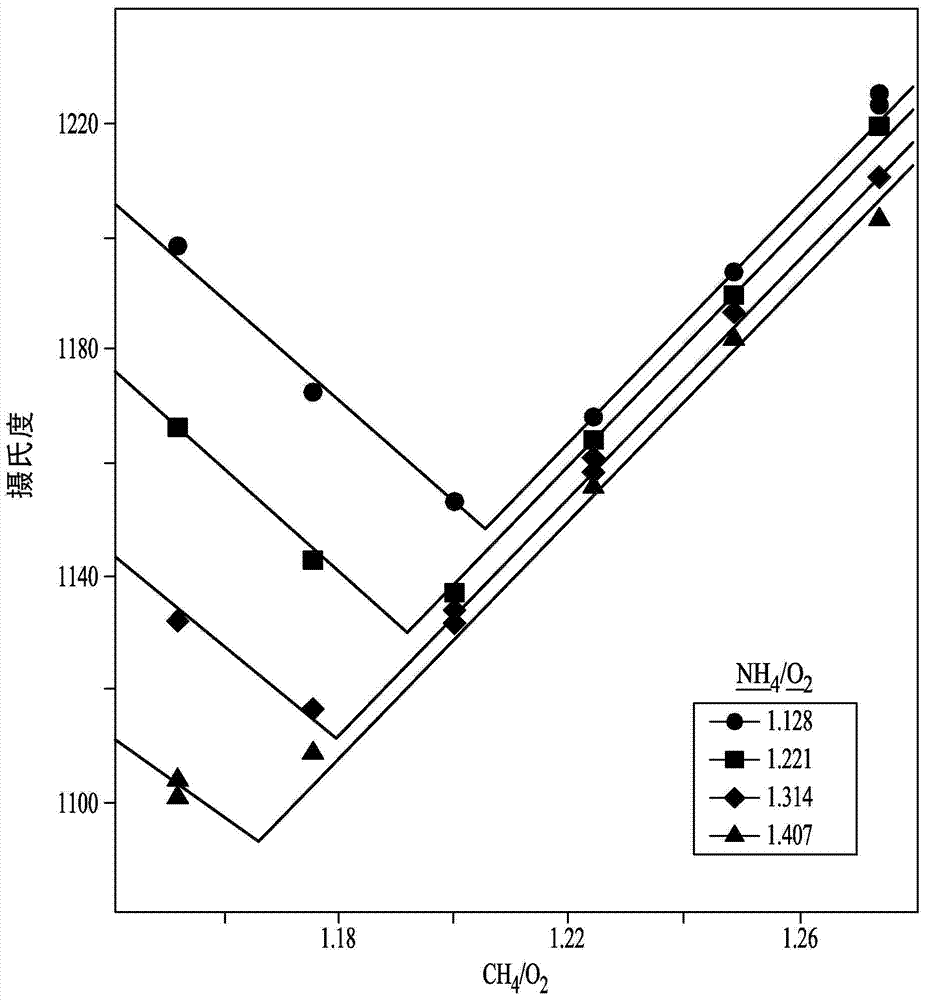
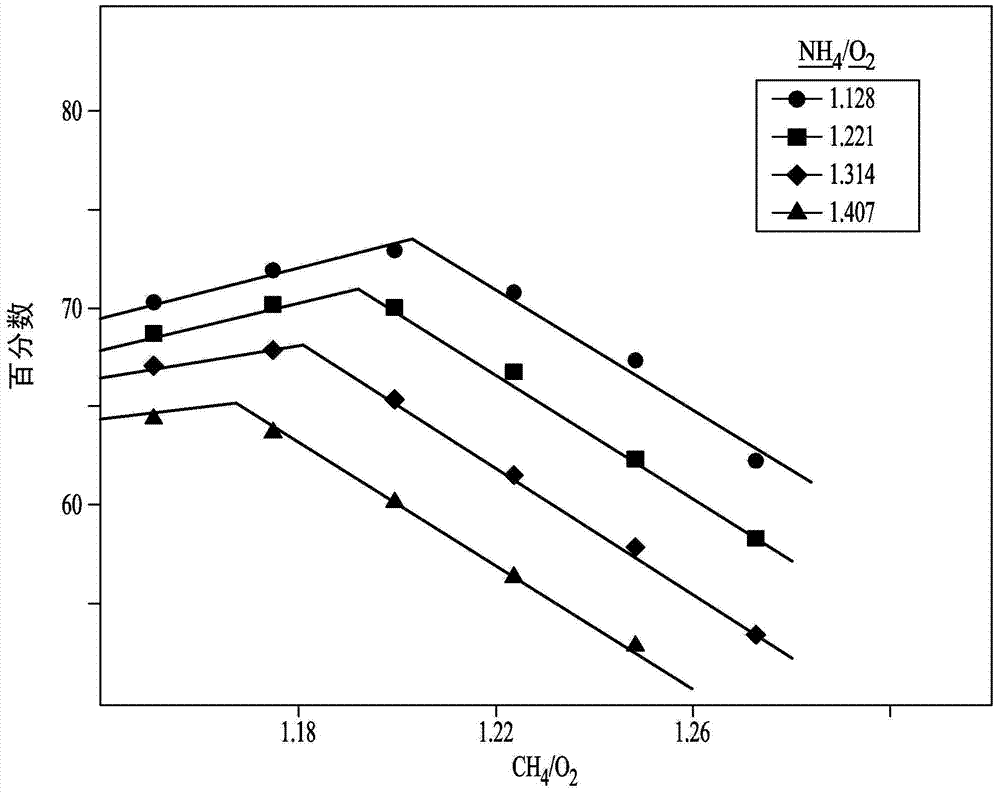
Variation of ammonia ratio in Andrussow process
A proportional, hydrogen cyanide technology, applied in the preparation/purification/separation, liquefaction, cyanide and other directions of hydrogen cyanide, can solve problems such as high variable costs, and achieve the effect of reducing costs and improving variable costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] Embodiment 1: change methane: ammonia molar ratio
[0077] Filtered ammonia, natural gas, and air or oxygen are fed into an Andrussow reactor and heated at a temperature in the range of about 1,050°C to about 1,200°C in the presence of a platinum-containing catalyst. A 4 inch inner diameter stainless steel reactor with a ceramic insulating liner inside was used for the pilot test. Forty pieces of 90 wt% Pt / 10 wt% Rh 40 mesh gauze from Johnson Matthey (USA) were loaded as catalyst bed. A perforated alumina sheet was used for the catalyst sheet support. Set the total flow rate at 2532 SCFH (standard cubic feet per hour). Some reactors are designed to use air as the oxygen source. Other Andrussow reactor designs use oxygen-enriched air and still others use oxygen as the oxygen-containing feed stream. However, the ratio of ammonia to methane can be varied in any of these methods to reduce cost. Natural gas can also be used instead of pure methane, especially if the nat...
Embodiment 2
[0091] Example 2: Impurity formation when unused methane is present
[0092] The Andrussow process was performed as described in Example 1, except that the amount of methane in the reaction vessel was varied. When the amount of methane in the reaction vessel increases beyond the level at which the methane is substantially consumed, some methane remains unreacted and exits through the reaction vessel. This unreacted methane is detected in the product stream and is referred to as "methane slip" or "methane loss".
[0093] Such as Figure 4 As shown in , the acetonitrile (CH 3 CN) The amount of impurities increases with the amount of methane leakage (unreacted methane). especially, Figure 4 Illustratively, when unreacted methane is greater than about 0.5 mole % methane per mole of HCN produced, significant amounts of acetonitrile begin to form, and increasing amounts of acetonitrile continue to form as the amount of unreacted methane increases.
[0094] During typical opera...
Embodiment 3
[0095] Embodiment 3: Use cost to change the molar ratio of methane and ammonia
[0096] This example illustrates the realization of cost savings for HCN production after evaluation of ammonia and methane costs and using this evaluation to adjust the methane:ammonia ratio in the Andrussow process.
[0097] The oxygen Andrussow process was performed as described in Example 1, but with a methane:ammonia ratio of about 0.8.
[0098] The average total percentage cost of methane for one week is X and the average total percentage cost of ammonia for the same week is Y. The cost of ammonia and methane is 90% of the total operating cost for the production of HCN (X+Y=90% of the total cost).
[0099] Since the methane:ammonia ratio is about 0.8, about 20% less methane than ammonia is used in this reaction. Thus, if the cost per mole of methane is about 0.01 of the cost per mole of ammonia, the total methane cost (X) is 0.008 of the total ammonia cost (Y), and X=0.008(Y) or Y=X / 0.008. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com