Reduction of organonitrile impurity levels in HCN from an oxygen Andrussow process
A technology of oxygen Andrussow method and organic nitrile, which is applied in the direction of inorganic chemistry, chemical instruments and methods, preparation/purification/separation of hydrogen cyanide, etc., which can solve the problems of increased possibility of HCN polymerization and degradation of tower performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
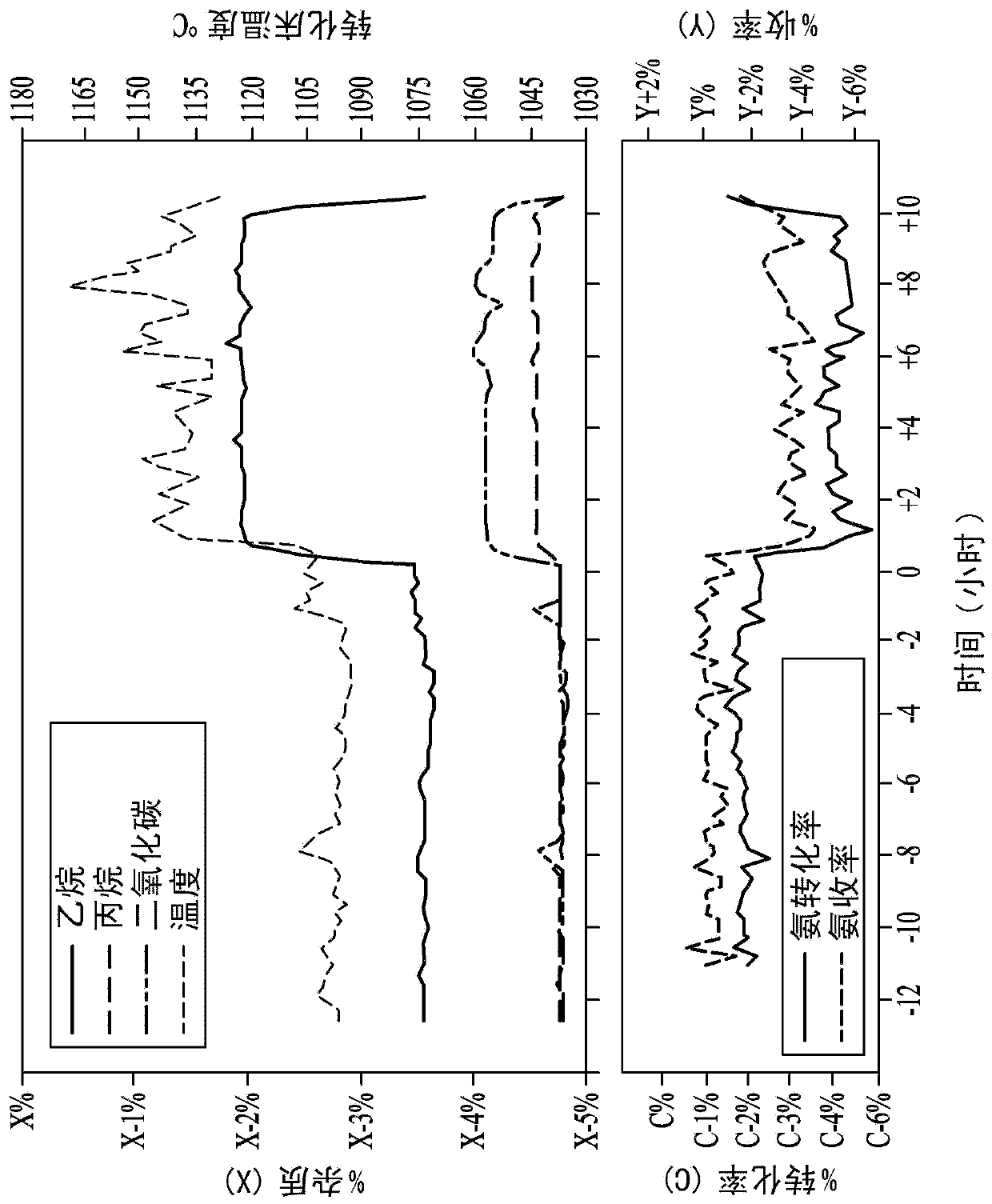
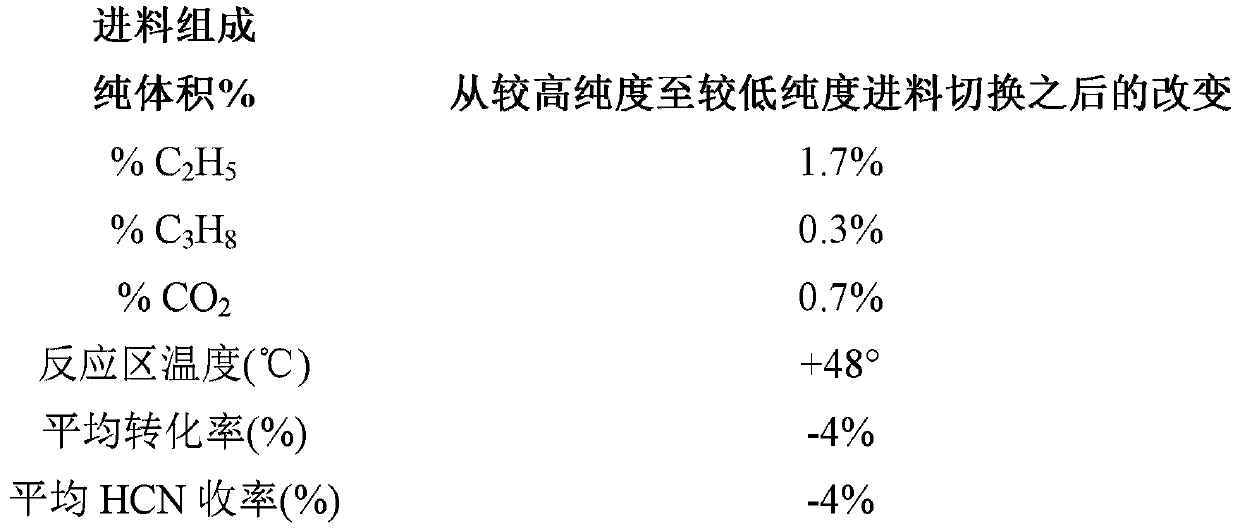
[0040] Example 1 details observations made during a period of time during operation of an oxygen Andrussow reactor during which changes in hydrocarbon composition were found to result in process temperature and product changes.
[0041] A 4 inch inner diameter stainless steel reactor with a ceramic insulating liner inside was used for the pilot test. Forty pieces of 90 wt% Pt / 10 wt% Rh 40 mesh gauze from Johnson Matthey (USA) were loaded as catalyst bed. A perforated alumina sheet was used for the catalyst sheet support. Set the total flow rate at 2532 SCFH (standard cubic feet per hour). In a simulated production sequence, three reactors were used in an oxygen Andrussow reaction plant to produce hydrogen cyanide from a reaction mixture of about 34 mole percent methane, about 37 mole percent ammonia, and about 27 mole percent oxygen in the presence of a platinum catalyst . The gaseous product stream from the reactor contained about 17 mole % hydrogen cyanide, about 6 mole %...
Embodiment 2
[0048] A 4 inch inner diameter stainless steel reactor with a ceramic insulating liner inside was used for the pilot test. Forty pieces of 90wt%Pt / 10wt%Rh 40 mesh gauze from Johnson Matthey (USA) were loaded as catalyst beds. A perforated alumina sheet was used for the catalyst sheet support. Set the total flow rate at 2532 SCFH (standard cubic feet per hour). In a simulated production sequence, three reactors were used in an oxygen Andrussow reaction plant to produce hydrogen cyanide from a reaction mixture of about 34 mole percent methane, about 37 mole percent ammonia, and about 27 mole percent oxygen in the presence of a platinum catalyst . The gaseous product stream from the reactor contained about 17 mole % hydrogen cyanide, about 6 mole % unreacted ammonia, about 35 mole % hydrogen, about 6 mole % CO, and about 34 mole % H 2 O, based on the reacted NH 3 With an overall yield of approximately 82% (on a molar basis). In the continuous oxygen Andrussow process carried...
Embodiment 3
[0050] A 4 inch inner diameter stainless steel reactor with a ceramic insulating liner inside was used for the pilot test. Forty pieces of 90wt%Pt / 10wt%Rh 40 mesh gauze from Johnson Matthey (USA) were loaded as catalyst beds. A perforated alumina sheet was used for the catalyst sheet support. Set the total flow rate at 2532 SCFH (standard cubic feet per hour). In a simulated production sequence, three reactors were used in an oxygen Andrussow reaction plant to produce hydrogen cyanide from a reaction mixture of about 34 mole percent methane, about 37 mole percent ammonia, and about 27 mole percent oxygen in the presence of a platinum catalyst . The gaseous product stream from the reactor contained about 17 mole % hydrogen cyanide, about 6 mole % unreacted ammonia, about 35 mole % hydrogen, about 6 mole % CO and about 34 mole % H 2 O, based on the reacted NH 3 With an overall yield of approximately 82% (on a molar basis). In the continuous oxygen Andrussow process carried ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com