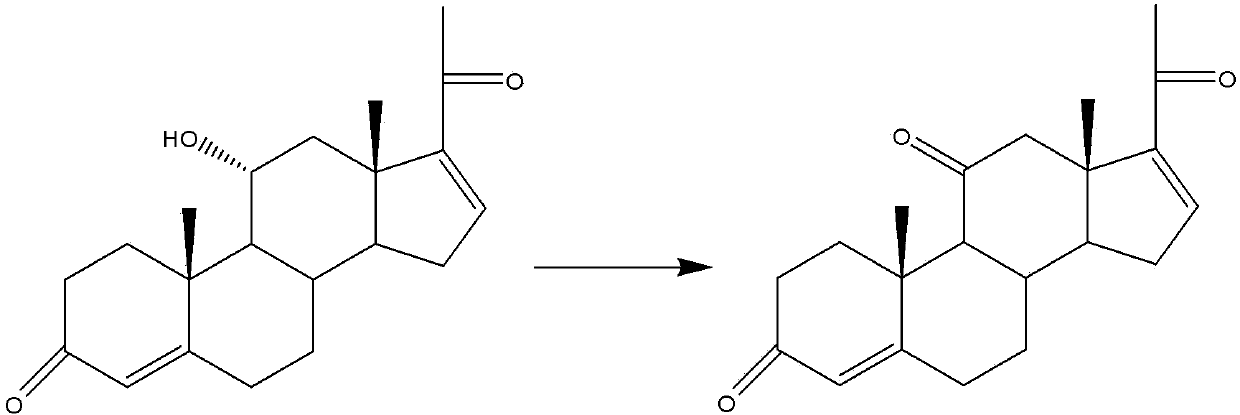
Selective oxidation method for hexa(hepta)decene-pregna-11-alpha-hydroxy
A technology of pregnan and diene, which is applied in the field of selective oxidation of 16(17) enepregnant 11α hydroxyl group, can solve the problems of green color of oxidation products and excessive heavy metals, difficult and easy to filter out, and inability to eradicate chromium ions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Pregna-4,16(17)-diene-3,20-diketone-11α-hydroxyl 10g (content 96.8%, maximum impurity <2%), dimethyl sulfoxide 30g in 100ml chloroform at -15°C , dicyclohexylcarbodiimide 5g for reaction, after the substrate content was detected by high performance liquid phase <1%, adding pyridine to adjust the pH to 8.5-10, and generating pregna-4,16 (17) by high performance liquid phase detection -diene-3,11,20-trione, concentrated under reduced pressure to no chloroform, cooled to 0°C, diluted with 100ml of 0°C water under stirring, and filtered to obtain pregna-4,16(17)-diene- 9.91g of 3,11,20-triketone, the content is 96.2%, and the maximum impurity is <2%.
Embodiment 2
[0019] Pregnant-4,16(17)-diene-3,20-diketone-11α-hydroxyl 10g in 50ml chloroform at -20°C (98.5% content, maximum impurity <2%), dimethylmethylene 6g of sulfone and 8g of phenyl phosphate dichloride were reacted. After the substrate content was detected by high performance liquid phase <1%, pyridine was added to adjust the pH to 8.5-10, and pregna-4, 16 (17 )-diene-3,11,20-trione, concentrated under reduced pressure to no chloroform, cooled to 0°C, diluted with 100ml of 0°C water under stirring, and filtered to obtain pregna-4,16(17)-diene -3,11,20-triketone 9.86g, content 98.1%, maximum impurity <2%.
Embodiment 3
[0021] Pregna-4,16(17)-diene-3,20-diketone-11α-hydroxyl 10g (content 97.4%, maximum impurity <2%), dimethyl sulfoxide 10g in 70ml chloroform at -30°C , N-chlorosuccinimide 30g reacted, after the substrate content was detected by high performance liquid phase <1%, triethylamine was added to adjust the pH to 8.5-10, and pregna-4,16 was generated by high performance liquid phase detection After (17)-diene-3,11,20-trione, add 15ml of water, shake, and then separate layers, take the chloroform layer, discard the water layer, if so, concentrate the chloroform layer under reduced pressure for 3 times, until the basic After chloroform-free, recrystallize with methanol, cool to 0°C for 2 hours, and filter to obtain 9.21 g of pregna-4,16(17)-diene-3,11,20-trione with a content of 99.1%. Maximum impurity <1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com