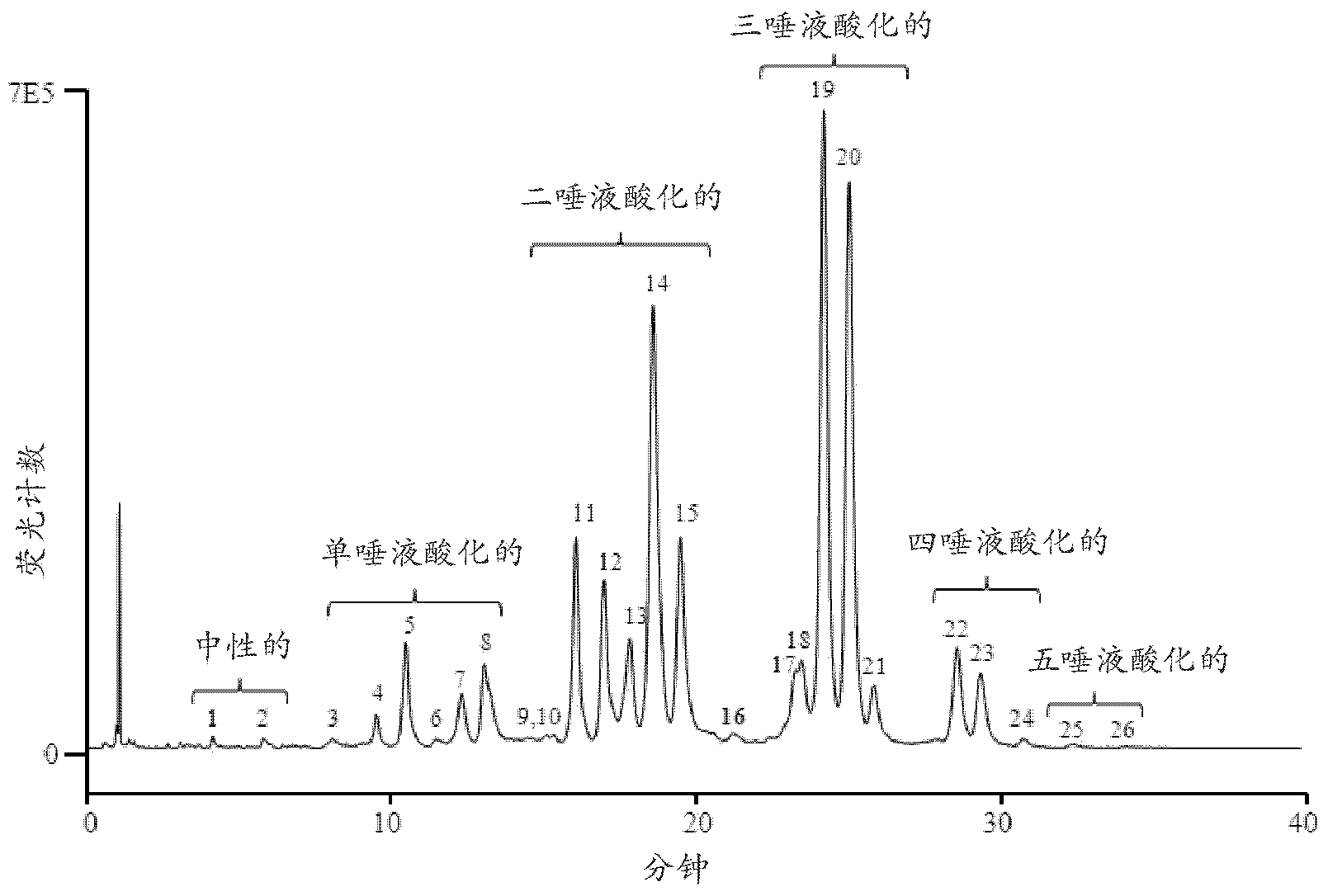
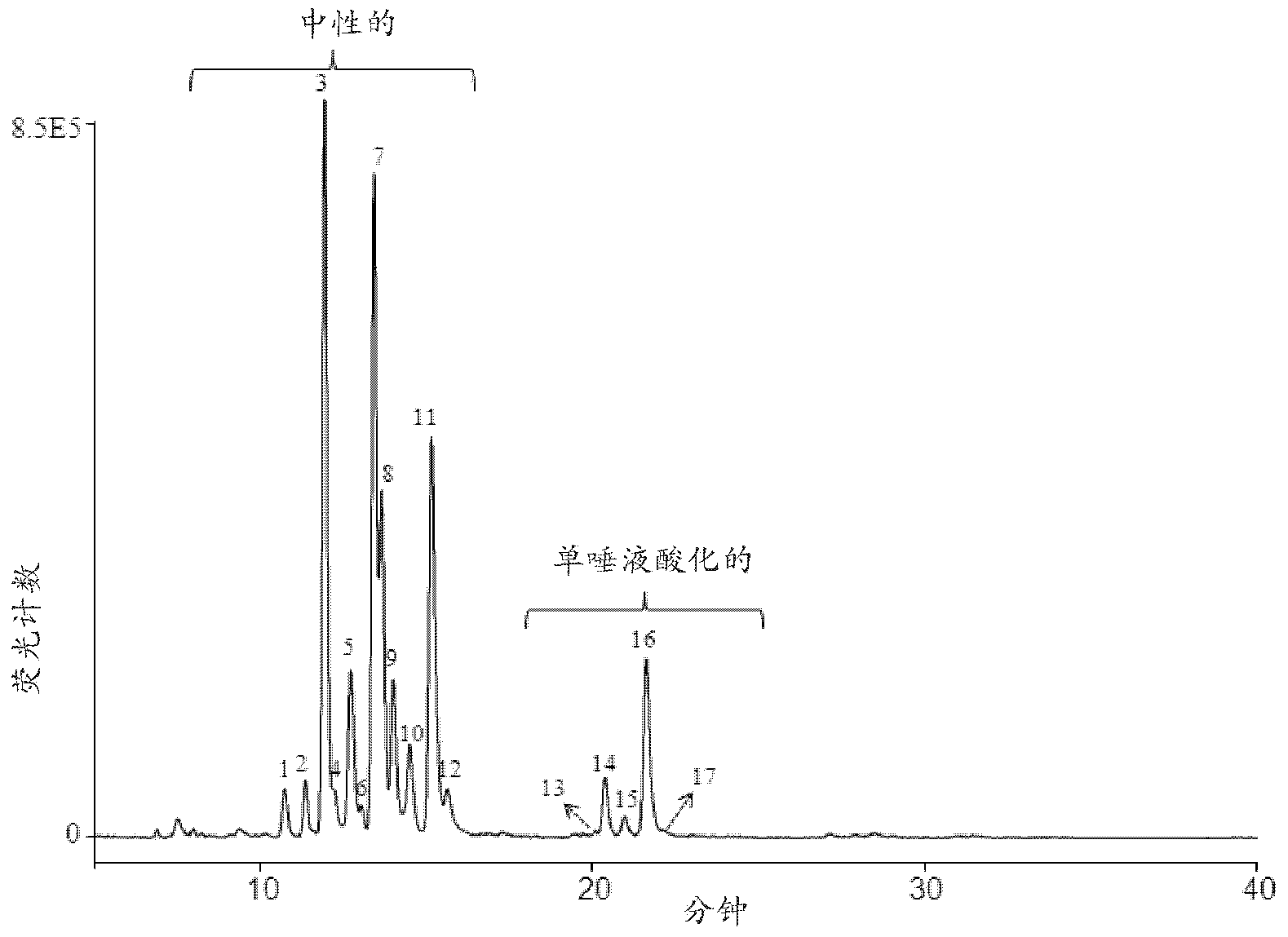
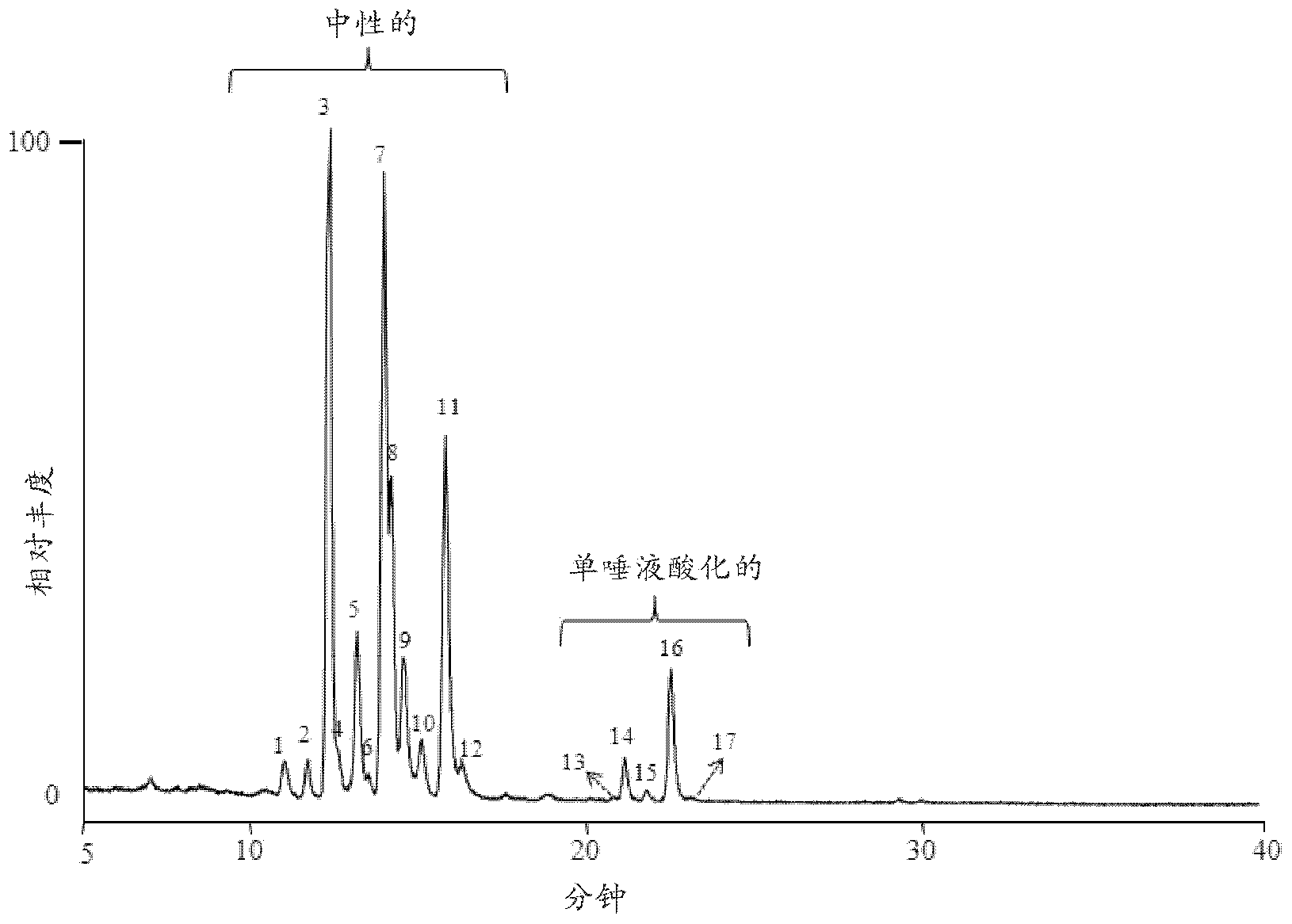
Separation Of Glycans By Mixed-mode Liquid Chromatography
A glycan and chromatography technology, applied in the field of separating glycans by mixed-mode liquid chromatography, can solve problems such as poor resolution and achieve the effect of excellent resolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0167] Example 1. Preparation of Compound 10
[0168] Dimethylamine (2) was mixed with excess triethylamine (2.0 equivalents) in anhydrous CH 2 Cl 2 Mix in medium and maintain between about 0°C and about 5°C for 20 minutes. Add one dropwise in CH 2 Cl 2 10-undecenoyl chloride (1) (1.0 equiv) in , and the mixture was stirred at ambient temperature for 12 hours. The reaction mixture was washed with water, washed with Na 2 SO 4 Dry and remove the solvent in vacuo to yield compound 3. An excess of methyldiethoxysilane (4) (10 equivalents) was added to compound 3, followed by a catalyst solution (0.1 mol %) (eg, hexachloroplatinic acid in minimal ethanol). After stirring at 50 °C for 24 hours, residual silane and solvent were removed in vacuo to provide compound 10. Figure 7 .
example 2
[0169] Example 2. Preparation of Compound 11
[0170] A solution of 11-bromo-1-undecene (5) at 5 °C in THF was added dropwise to a solution of dimethylamine (2) (10 equiv) in THF and the Stirring was continued for 12 hours. The volatiles were removed in vacuo and the residue partitioned in CH 2 Cl 2 with H 2 Between O, use Na 2 SO 4 Drying, which was followed by removal of solvent in vacuo afforded compound 6. An excess of methyldiethoxysilane (4) (10 equivalents) was added to compound 6, followed by a catalyst solution (0.1 mol%) (eg, hexachloroplatinic acid in a minimum of ethanol). After stirring at 50 °C for 24 hours, residual silane and solvent were removed in vacuo to afford silyl compound 11. Figure 7 .
example 3
[0171] Example 3. Preparation of Phase 21
[0172] Compounds 10 and 11 were mixed in toluene at a predetermined ratio. A predetermined amount of crude silica gel is then added to this solution while stirring until homogeneity is obtained. The reaction mixture was kept at reflux for 3 days. The resulting mixture was filtered, washed with acetone, and dried under vacuum at 50° C. for 5 hours to give the functionalized silica of composition 21 . A capping step with trialkylchlorosilanes and / or dialkyldichlorosilanes can also be combined to produce a packing material for chromatographic separations. Figure 7 .
[0173] In an exemplary experimental protocol, 18 g of Compound 10, 1 g of Compound 11, and 15 g of crude silica gel (particle size, 1.9-μm; pore size, Surface area, 225m 2 / g) was dispersed in 50 mL of toluene (anhydrous) in a 250-mL round bottom flask and mixed in toluene. A predetermined amount of crude silica gel is then added to this solution while stirring unt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com