Poly-crystal-form substance of lapatinib ditosylate solvate as well as preparation method and application thereof
A technology of di-p-toluenesulfonic acid and lapatinib, which is applied in the preparation of organic compounds, chemical instruments and methods, organic chemistry, etc., and can solve problems such as fluidity and static electricity difference
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0060] The present invention provides a preferred method for preparing the polymorphic form of lapatinib di-p-toluenesulfonate N-methylpyrrolidone solvate according to the present invention, comprising the steps of:
[0061] (1) A mixture of lapatinib di-p-toluenesulfonate crude product and N-methylpyrrolidone is provided, the temperature of the mixture is raised to 35-50°C (preferably 35-40°C), and stirred until dissolved, thereby forming a mixture containing N-methylpyrrolidone solution of lapatinib di-p-toluenesulfonate;
[0062] (2) keep the temperature of step (1) (such as 35-50 ° C; preferred 35-40 ° C), in the N-methylpyrrolidone solution containing lapatinib di-p-toluenesulfonate in step (1) Add the eluent dropwise to precipitate crystals;
[0063] (3) Separating the separated crystals to obtain the polymorphic form of lapatinib di-p-toluenesulfonate N-methylpyrrolidone solvate according to the present invention.
[0064] In another preference, the step (2) is: at 35...
Embodiment 1 2
[0079] Example 1 Preparation of lapatinib di-p-toluenesulfonate NMP solvate crystal form A
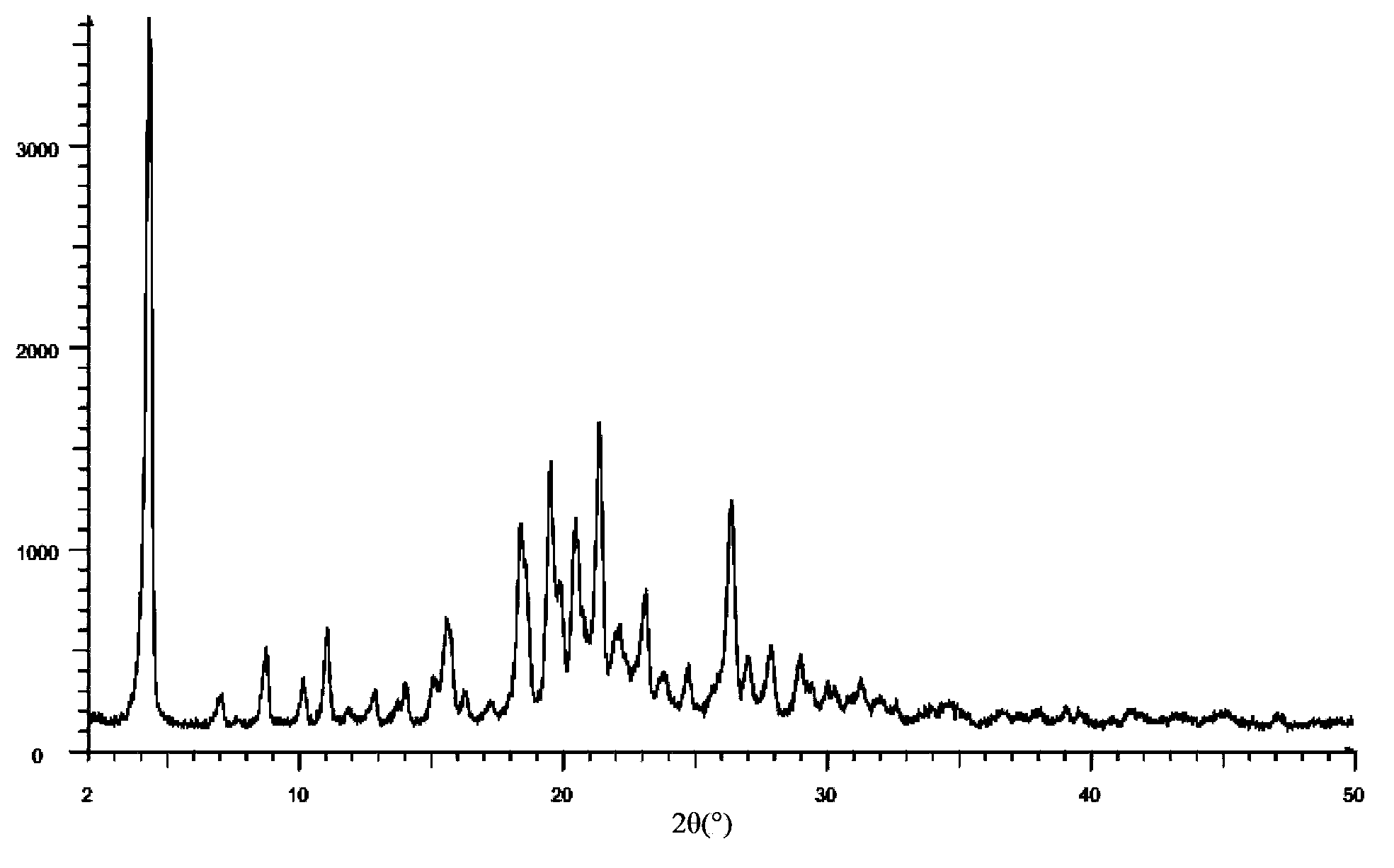
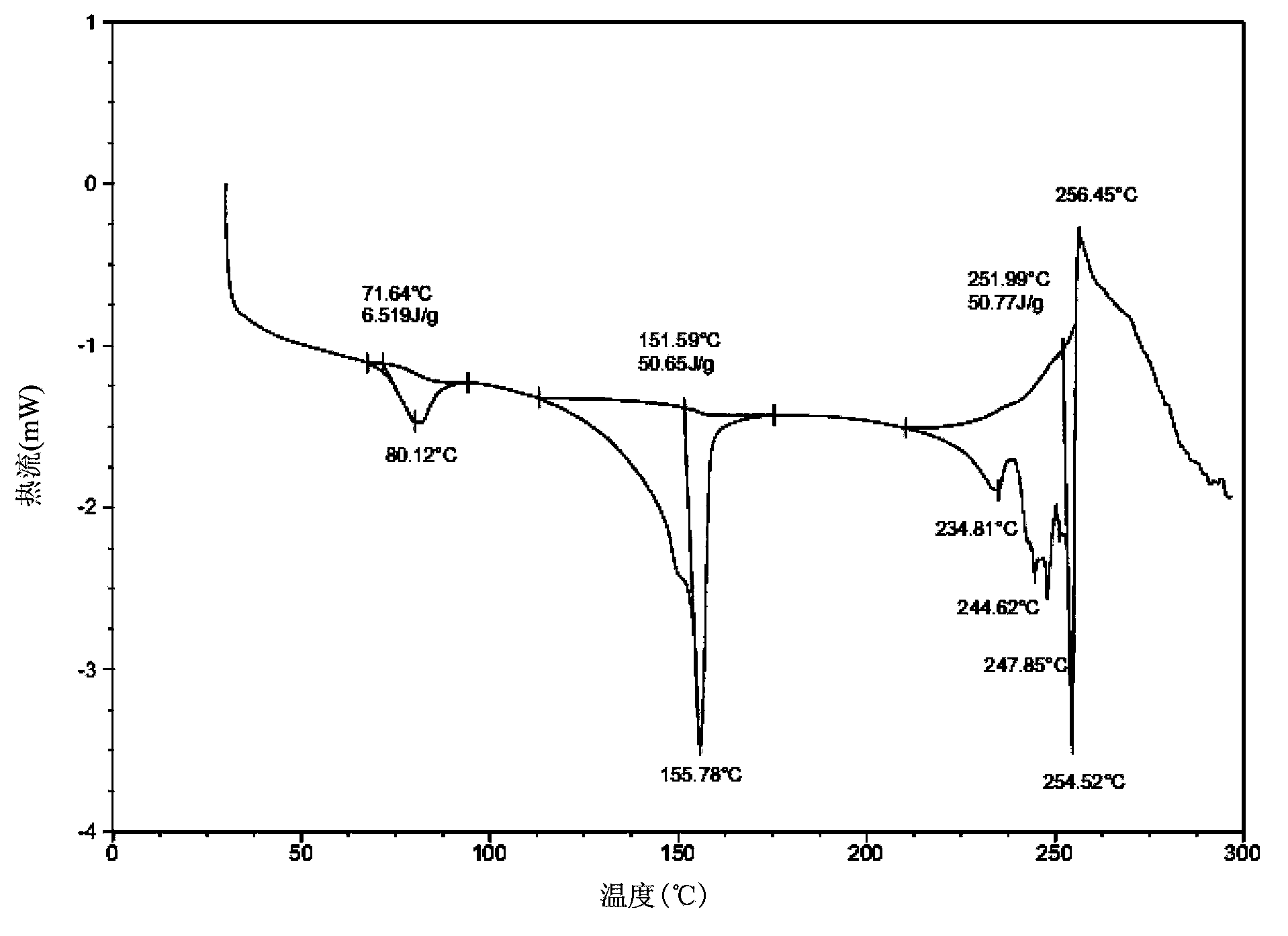
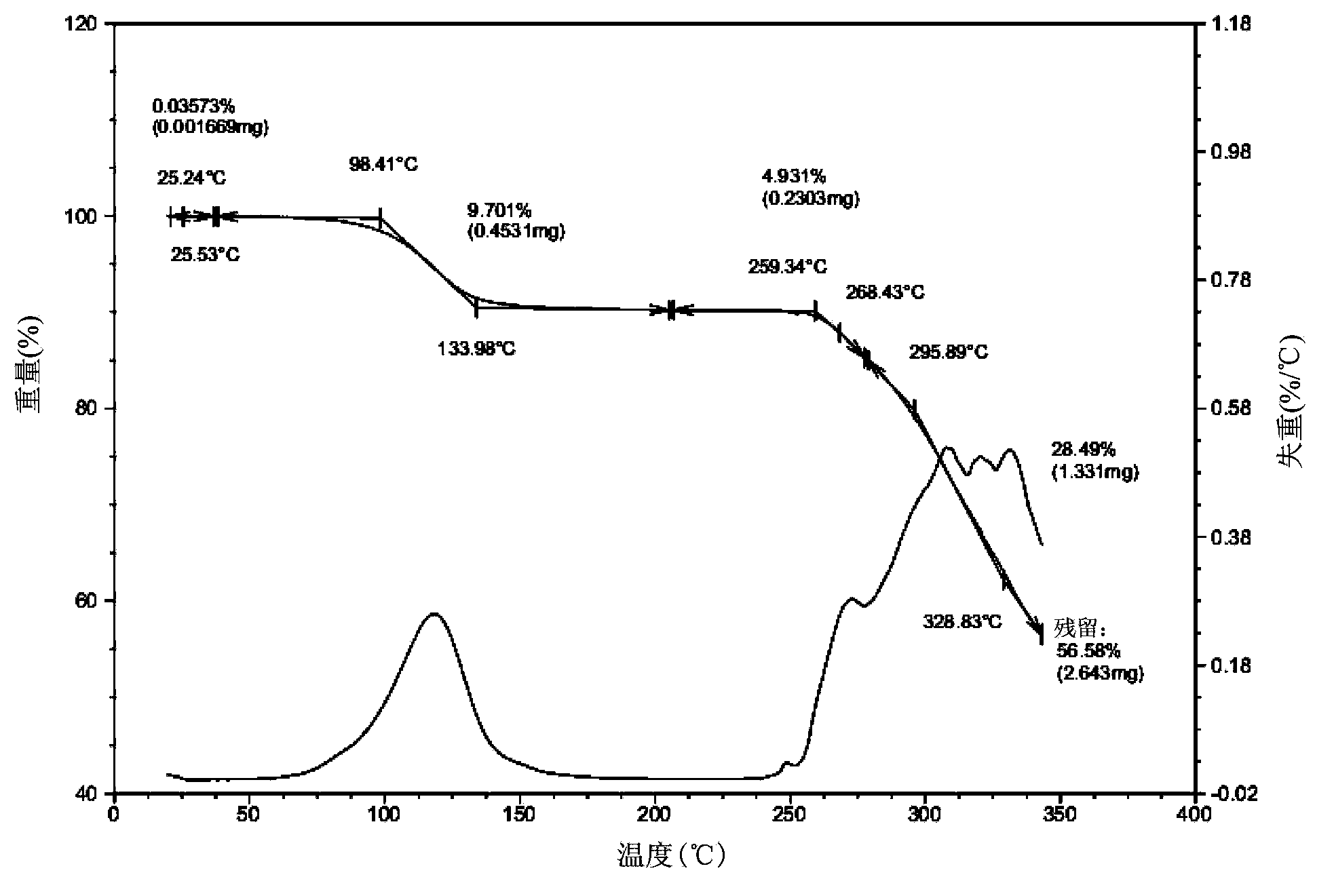
[0080] Add 20g lapatinib di-p-toluenesulfonate crude product and 250ml NMP in reaction bottle, be warming up to 35-40 ℃, stir, until solid dissolves completely, obtain bright yellow solution, keep stirring for 30 minutes. Then maintain the temperature of solution to be 35-40 DEG C, drip acetone 600ml with the speed of 2ml / min, begin to separate out crystal. After the dropwise addition, slowly drop to 15°C. Filter and wash the filter cake 2-3 times with 90ml acetone. Vacuum drying at 45° C. for 12 hours yielded 21.3 g of light yellow di-p-toluenesulfonate lapatinib NMP solvate crystals. The HPLC detection purity was 99.6%, and the crystal form purity was about 100%. Its powder X-ray diffraction pattern is basically as figure 1 As shown, the differential thermal scanning spectrum is basically as figure 2 As shown, the thermogravimetric analysis is basically as image 3 shown. ima...
Embodiment 2 2
[0081] Example 2 Preparation of lapatinib di-p-toluenesulfonate NMP solvate crystal form A
[0082] Add 10g lapatinib di-p-toluenesulfonate crude product and 180ml NMP in reaction bottle, be warming up to 35 ℃, stir, until solid dissolves completely, keep stirring for 1 hour. Then maintain the temperature of solution to be 35 DEG C, drip acetone 550ml with the speed of 2.5ml / min, begin to separate out crystal. After the dropwise addition, slowly drop by 10°C. Filter and wash the filter cake 2-3 times with 30ml acetone. Vacuum drying at 55° C. for 8 hours gave 9.6 g of pale yellow lapatinib di-p-toluenesulfonate NMP solvate crystals. The HPLC detection purity was 99.8%, and the crystal form purity was about 100%. Its XRD figure, DSC figure, TGA figure are basically consistent with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com