A kind of flutamide sustained-release preparation and preparation method thereof
A sustained-release preparation, flutamide technology, applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problem of unbalanced release, rapid absorption and slow excretion, and difficulty in achieving sustained release And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
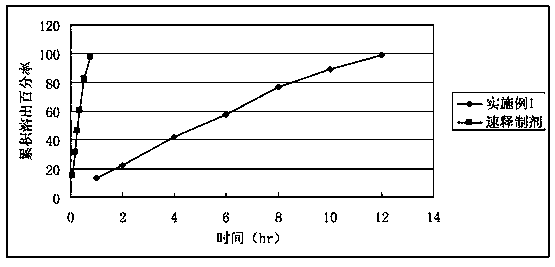
Embodiment 1
[0033] Flutamide 500 Ethyl cellulose 20g Polyoxyethylene N10 84 Polyoxyethylene N12K 36 Polyvinylpyrrolidone 5 Magnesium stearate 5, the binder used is 6% polyvinylpyrrolidone solution prepared with 95% ethanol
[0034] The preparation process is as follows:
[0035] Polyoxyethylene and polyethylene glycol are mixed evenly, and flutamide is added in equal increments, mixed evenly, added with 6% polyvinylpyrrolidone alcohol solution to granulate, dried, granulated, added lubricant, mixed evenly, and compressed into tablets.
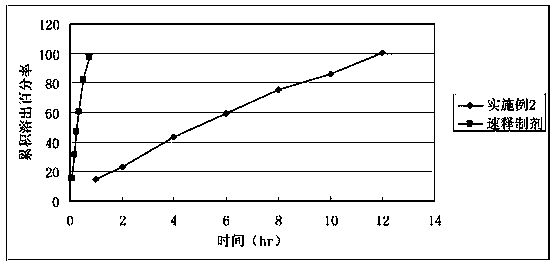
Embodiment 2
[0037] Flutamide 500g Microcrystalline cellulose 20g Polyoxyethylene N10 60g Polyoxyethylene N12K 40g Polyvinylpyrrolidone 5 g Stearic acid 6 g Magnesium stearate 5 g Among them, the binder used is 6% polyvinyl alcohol processed with 95% ethanol Vinylpyrrolidone solution.
[0038] The preparation process is as follows:
[0039] Raw and auxiliary materials are mixed evenly, 6% polyvinylpyrrolidone solution is added to granulate, dried, granulated, lubricant is added, mixed evenly, and tabletted.
Embodiment 3
[0041] Flutamide 500 g Polyoxyethylene 100 g Ethyl cellulose 20 g Polyvinylpyrrolidone 5 g Magnesium stearate 5g, the binder used is 6% polyvinylpyrrolidone solution prepared with 95% ethanol
[0042] The tablet preparation process is as follows:
[0043] Polyoxyethylene and ethyl cellulose are mixed evenly, and flutamide is added in equal increments, mixed evenly, added with 6% polyvinylpyrrolidone solution to granulate, dried, granulated, added lubricant, mixed evenly, and compressed into tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com