Synthesis method of drug intermediate flutamide
A technology of a body flutamide and a synthesis method, which is applied in the field of synthesis of a pharmaceutical intermediate flutamide, can solve problems such as decreased curative effect, and achieve the effects of sufficient exposure, high catalytic efficiency and stable catalytic activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
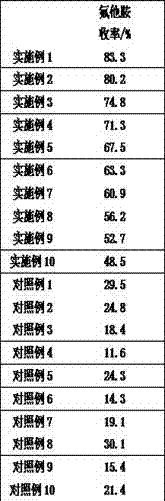
Examples
Embodiment 1
[0018] A kind of synthetic method of medicine intermediate flutamide, this method comprises the following steps:
[0019] Step 1. After adding 20ml of n-hexane into a 100ml three-necked flask, add 2.0g of catalyst and 3.22g of m-trifluoromethylaniline, start magnetic stirring, heat to 45°C, and drop 6.32g of isobutyric anhydride within 30min. Incubate for 1 hour, sample and analyze;
[0020] Step 2. Add 10ml of water to the reaction solution. After the addition, the temperature drops. Then add 10ml of 30% NaOH solution again. The temperature rises to above 50°C, then rises to 70°C, keeps warm for 15min, puts it in an ice machine, and cools down to 10°C. Filter and dry under vacuum at 60°C to obtain a white solid;
[0021] Step 3. Put the 100ml three-necked bottle equipped with mechanical stirring and a thermometer into the ice machine, add 23ml of concentrated sulfuric acid, stir, add 10.75g of m-isobutyramide trifluorotoluene, after the solid is dissolved, keep the temperatu...
Embodiment 2
[0031] Step 1. After adding 20ml of n-hexane into a 100ml three-necked flask, add 2.0g of catalyst and 1.61 m-trifluoromethylaniline, start magnetic stirring, heat to 45°C, add 6.32g of isobutyric anhydride dropwise within 30min, and keep warm 1h, sampling analysis; the rest of the steps are the same as in Example 1.
Embodiment 3
[0033] Step 1. After adding 20ml of n-hexane into a 100ml three-necked flask, add 2.0g of catalyst and 0.81 m-trifluoromethylaniline, start magnetic stirring, heat to 45°C, add 6.32g of isobutyric anhydride dropwise within 30min, and keep warm 1h, sampling analysis; the rest of the steps are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com