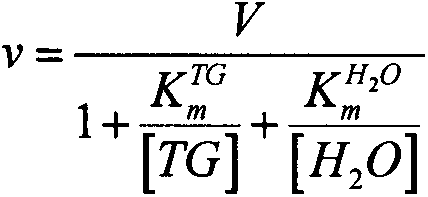Cyclophorase determination method for triglyceride in serum
A triglyceride and measurement method technology, which is applied in the field of determination including enzymes, can solve the problems of endogenous glycerol interference, etc., and achieve the effect of increasing reagent cost and high accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Composition of reagents:
[0061] a. Reagent I:
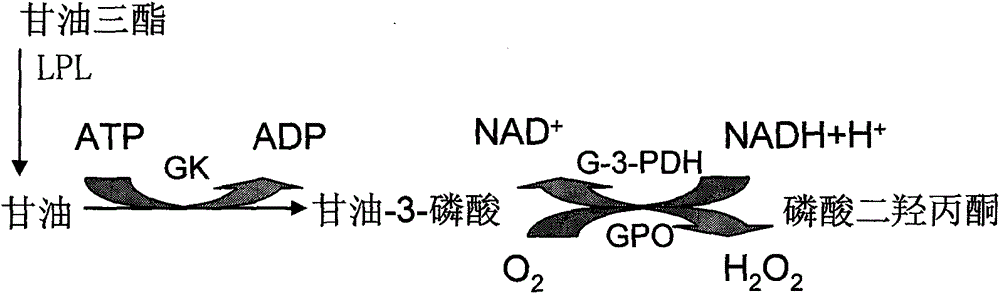
[0062] Reagent I Each liter of Tris-HCl buffer contains Tris200mmol, 2,4-dichlorophenol 2.0mmol, MgSO 4 12.0mmol, sodium cholate 4.0mmol, adenosine triphosphate 2.0mmol, glycerol kinase 130U, glycerol phosphate oxidase 1600U, peroxidase 1200U, 4-aminoantipyrine 2.0mmol, glycerol-3-phosphate dehydrogenase 1600U, NADH1 .0mmol, Proclin300 preservative 200μl.
[0063] b. Reagent II:
[0064] Reagent II contains Tris200mmol, lipoprotein lipase 1950U, Triton X-1000.12g, and Proclin300 preservative 200μl per liter of Tris-HCl buffer.
[0065] The pH value of the Tris-HCl buffer in the above reagent I and reagent II is 7.6±0.2.
[0066] c. Standard solution: 2.0mmol / L trioleate aqueous solution.
[0067] Among them, MgSO 4 It is an activator of glycerol kinase, Triton X-100 is a surfactant, Proclin-300 is a liquid high-efficiency preservative, and sodium cholate is a bacteriostatic agent.
Embodiment 2
[0069] Measurement procedure
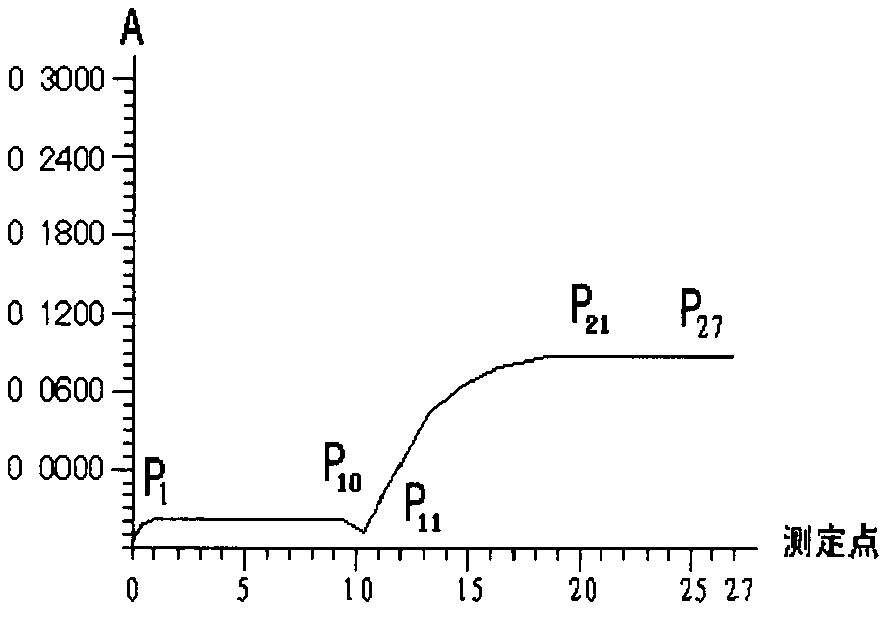
[0070] On the Japanese OLYMPUS AU2700 fully automated biochemical analyzer, the instrument automatically adds 1.5 μl of sample to 200 μl of reagent I and mixes it, incubates at 37°C for 3 minutes, adds 50 μl of reagent II and mixes it, and incubates at 37°C for 5 minutes. Detect at a wavelength of 500nm, and the instrument automatically calculates the TG result, see Table 1 for details.
[0071] Table 1 Automatic biochemical analyzer test conditions of the present invention
[0072]
[0073] response OD TG Calculated value = OD 2 -OD 1 ×[(SV+R 1 V 1 ) / (SV+R 1 V 1 +R 2 V 2 )]
[0074] Triglyceride concentration = F × OD TG
[0075] where OD TG is the absorbance produced by triglycerides. OD 1 is the absorbance measured after adding reagent I to the sample, OD 2 is the absorbance measured after adding reagent II to the sample, SV is the volume of serum, R 1 V 1 is the volume of reagent I, R 2 V 2 is the volume of reagent II. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com