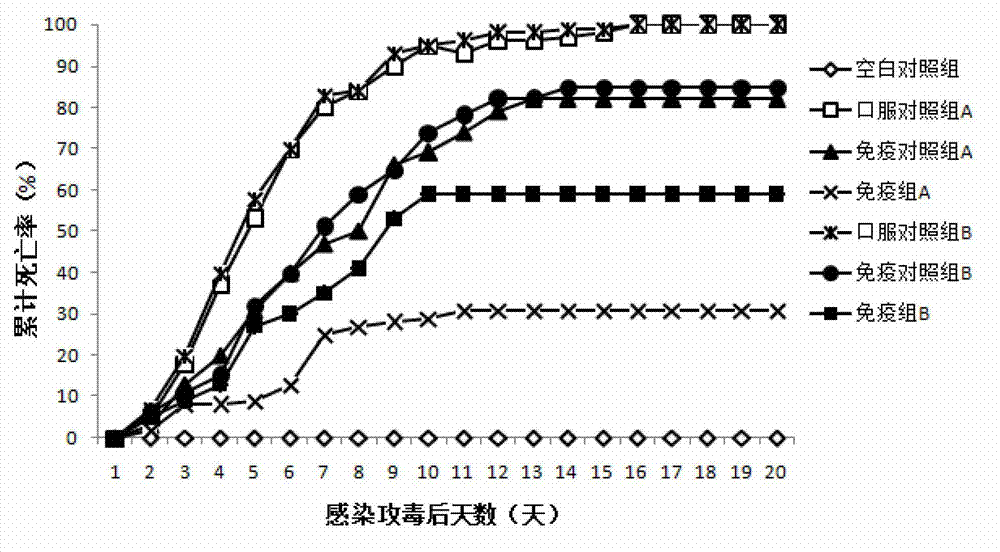
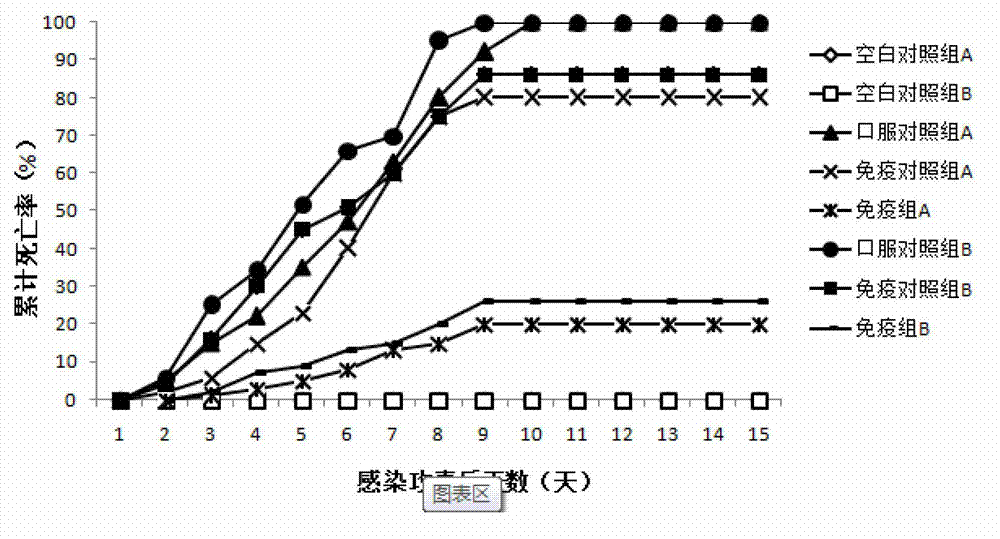
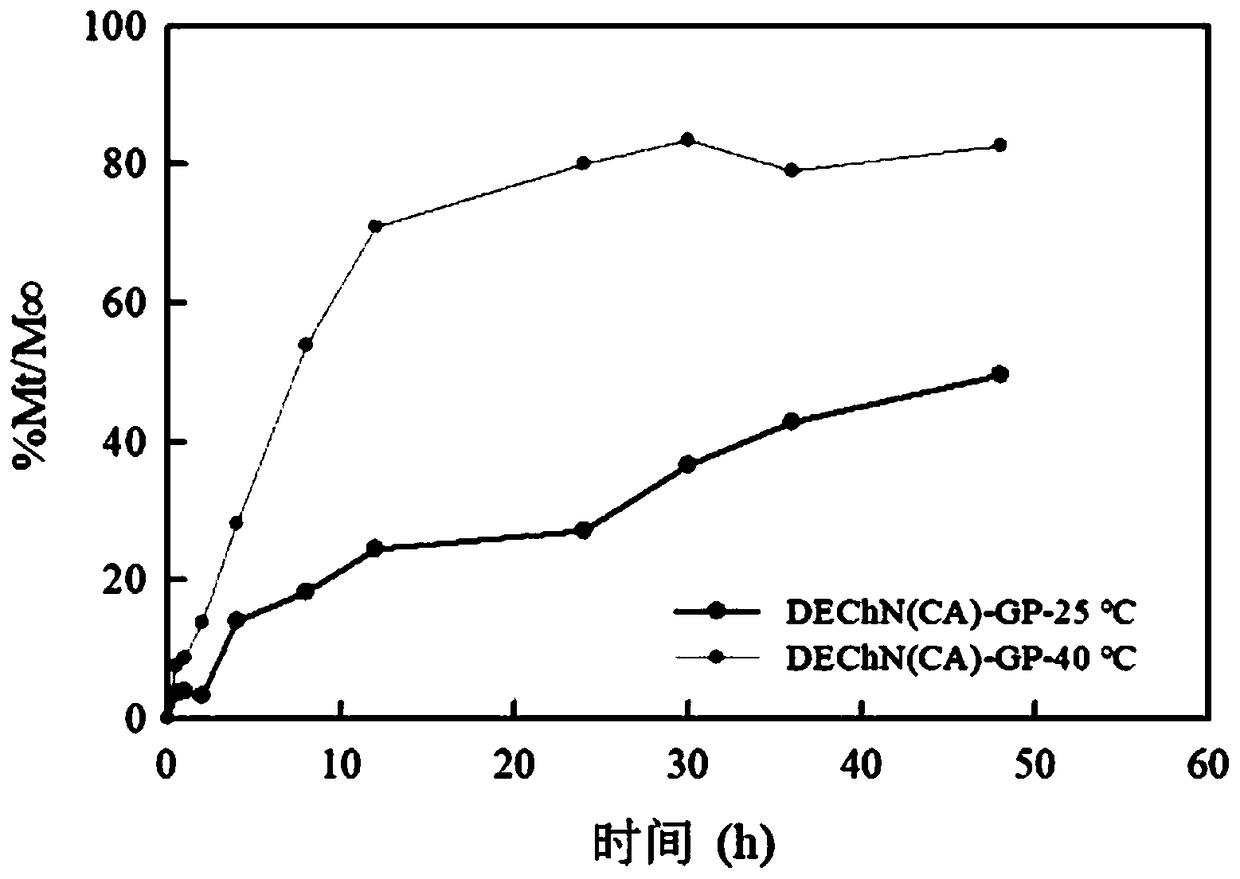
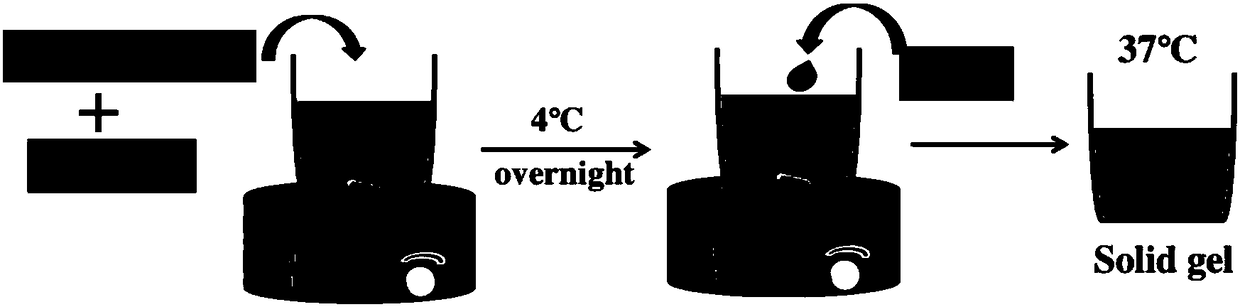
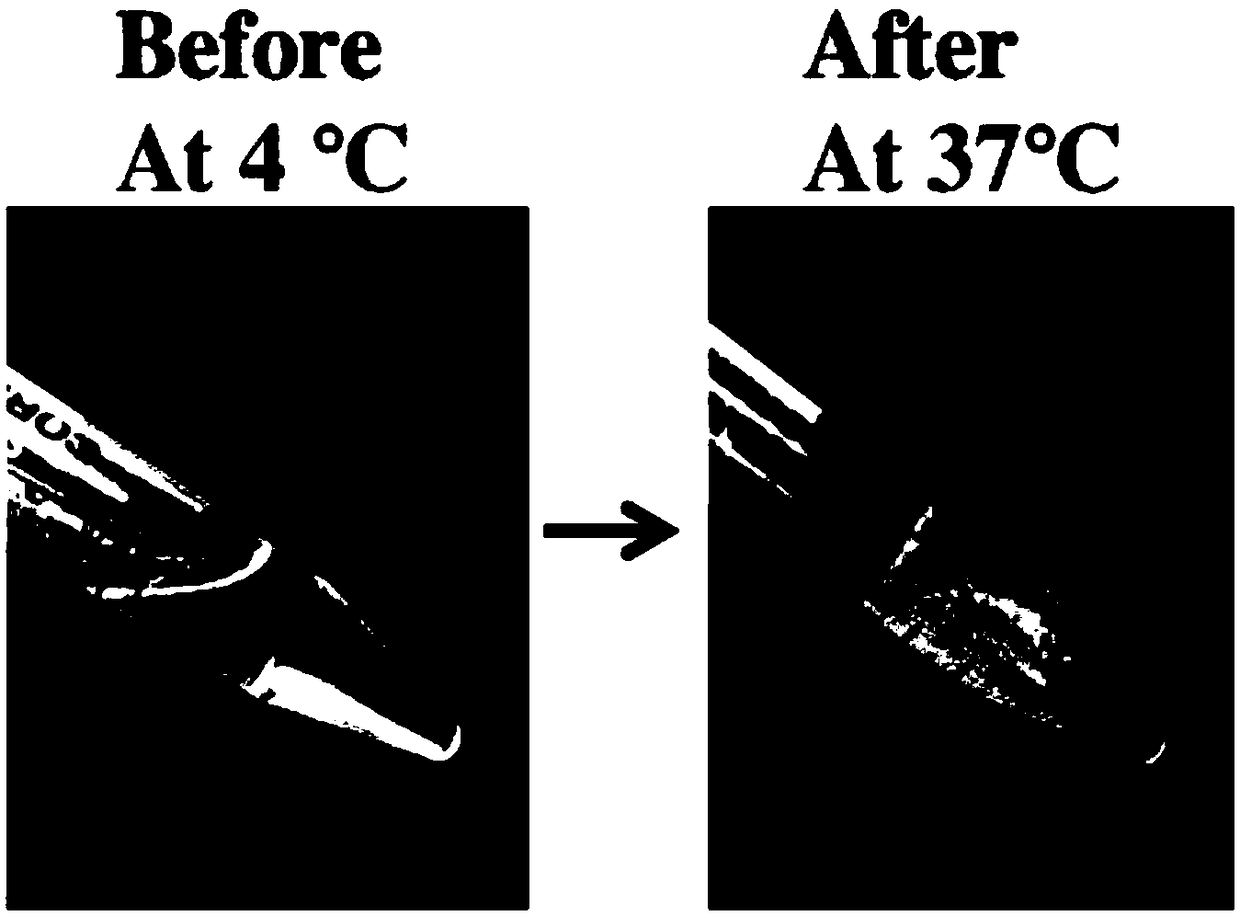
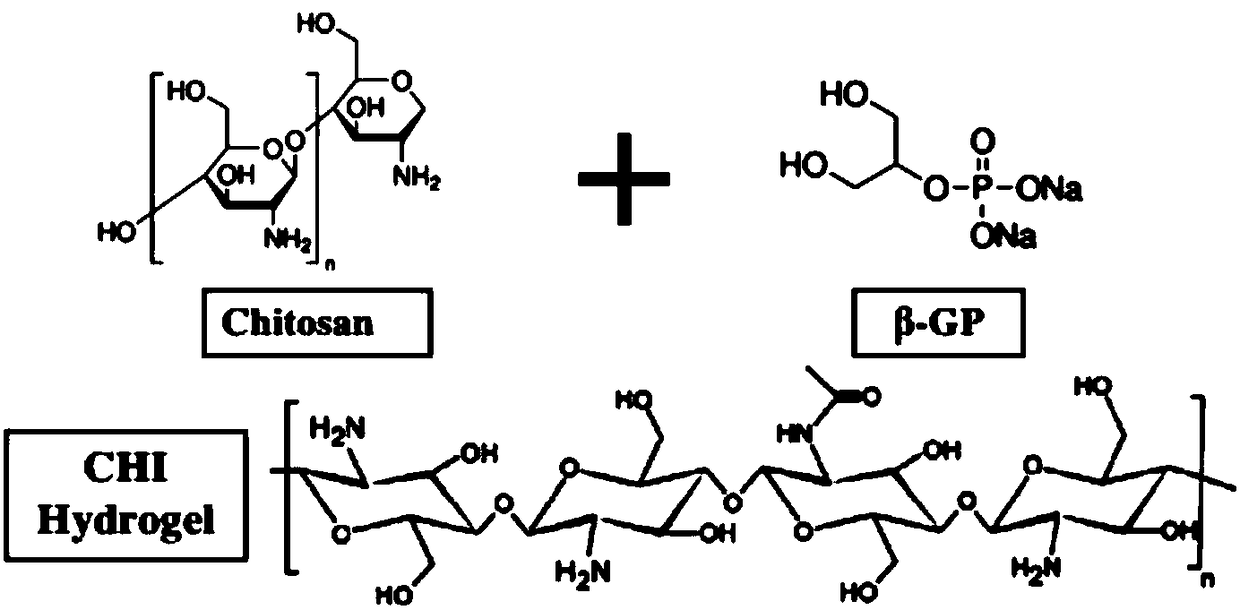
Patents
Literature
177 results about "Glycerol phosphate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Enhanced pyruvate to acetolactate conversion in yeast
ActiveUS20090305363A1Improve throughputReduction of pyruvate decarboxylase activityFungiTransferasesYeastCytosol
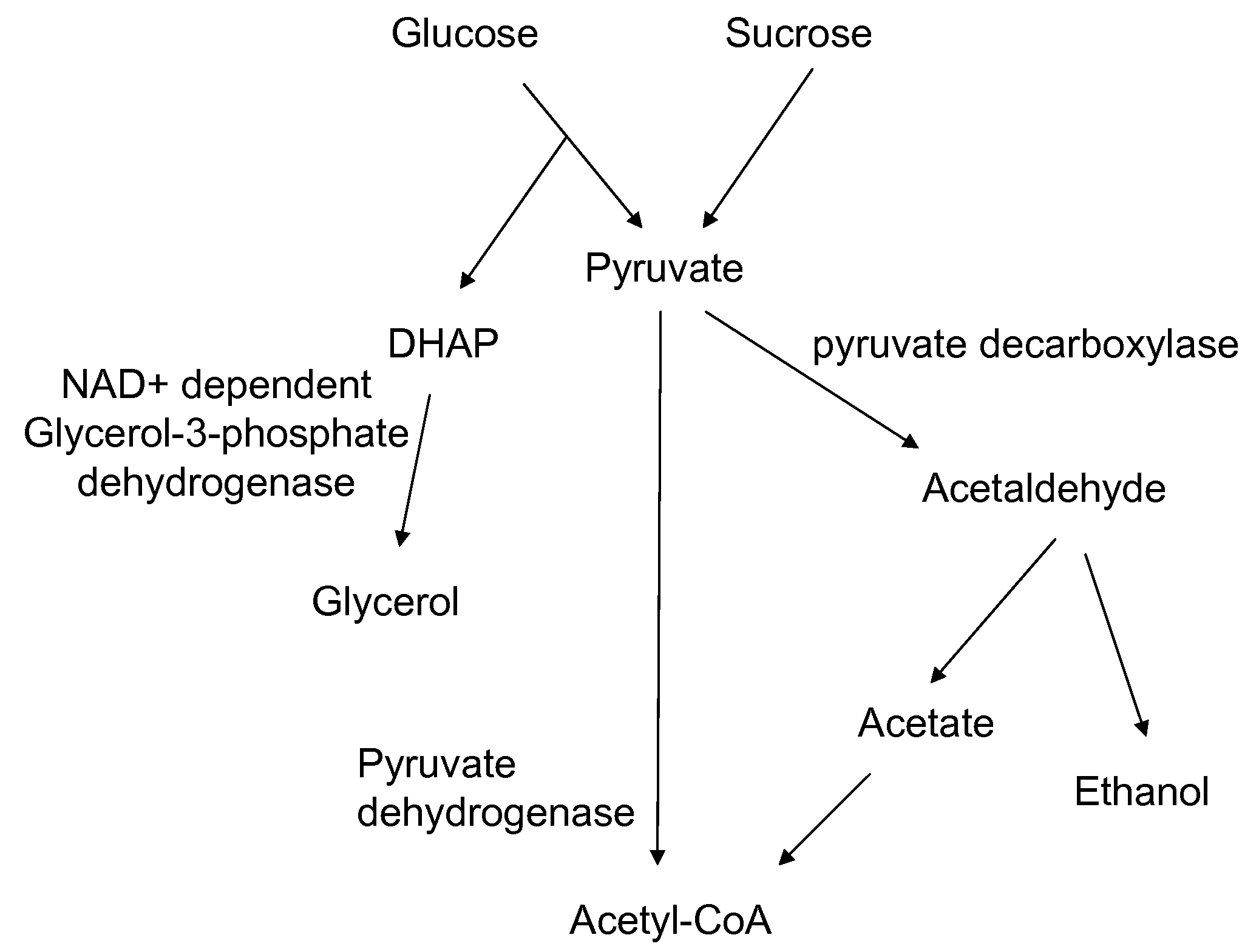
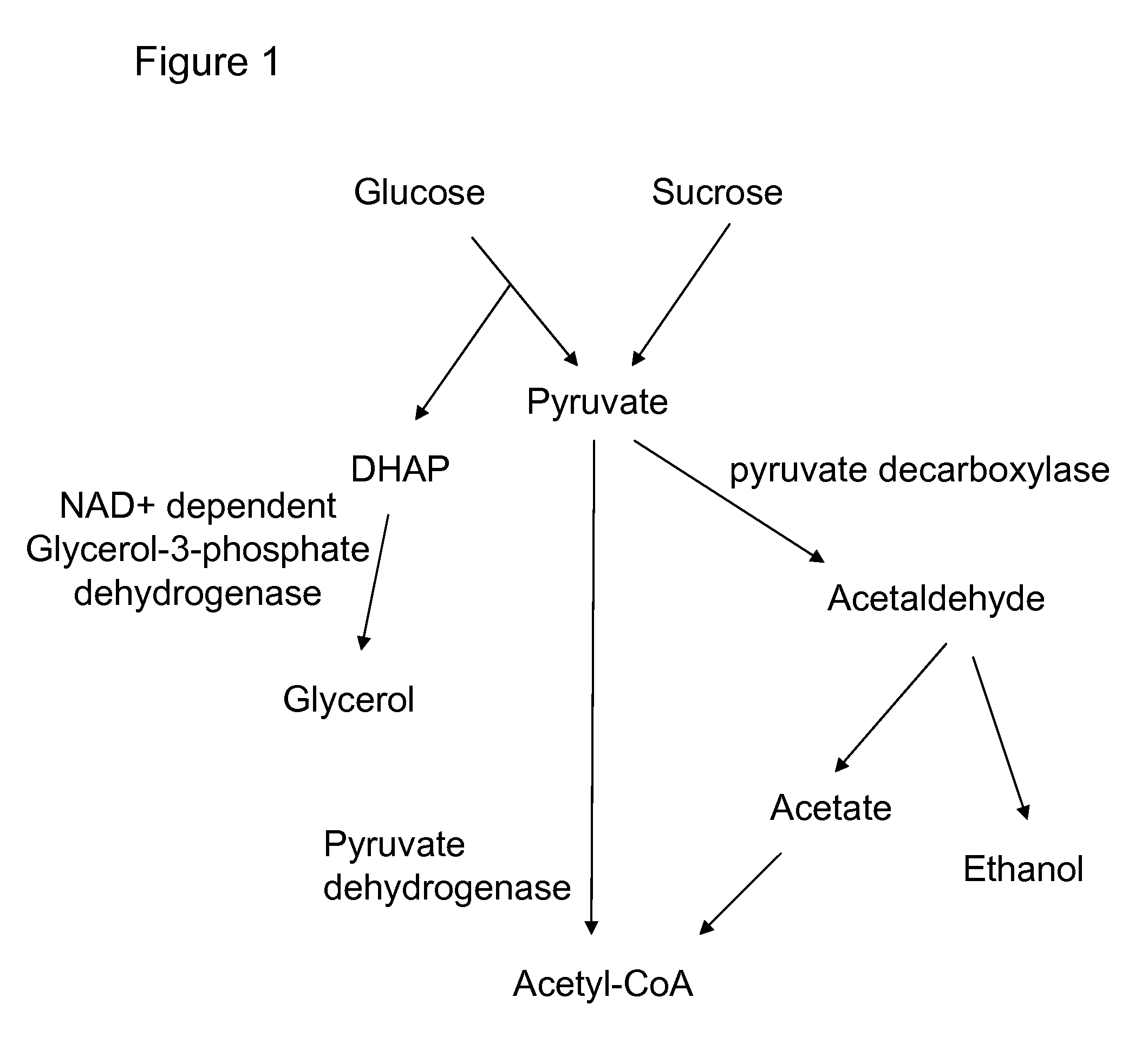
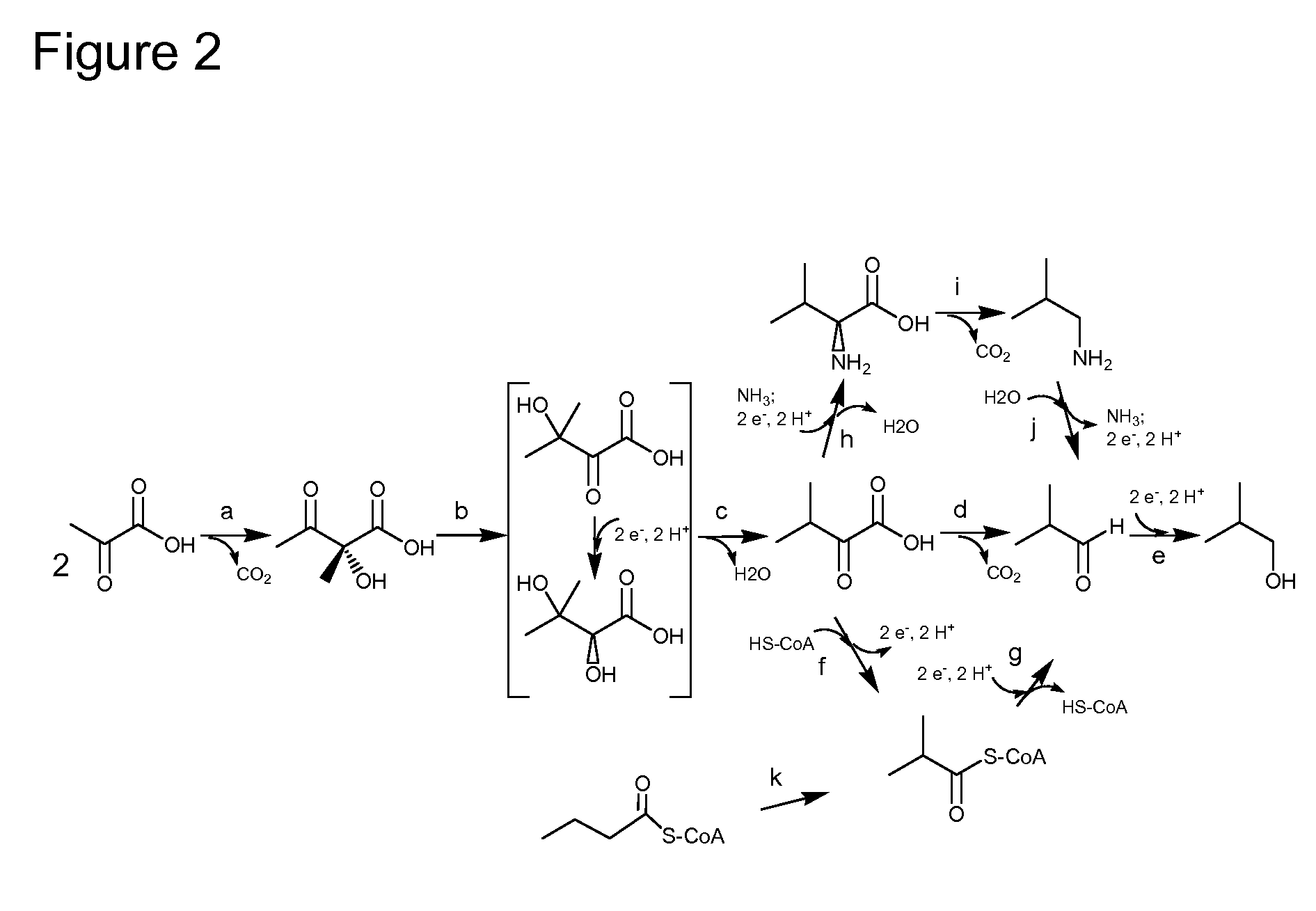
A high flux in conversion of pyruvate to acetolactate was achieved in yeast through expression of acetolactate synthase in the cytosol in conjunction with reduction in pyruvate decarboxylase activity. Additional manipulations to improve flux to acetolactate are reduced pyruvate dehydrogenase activity and reduced glycerol-3-phosphate dehydrogenase activity. Production of compounds having acetolactate as an upstream intermediate benefit from the increased conversion of pruvate to acetolactate in the described strains.
Owner:GEVO INC
Glyceraldehyde-3-phosphate dehydrogenase and phosphoglycerate mutase regulatory sequences for gene expression in oleaginous yeast
The regulatory sequences associated with the Yarrowia lipolytica glyceraldehyde-3-phosphate dehydrogenase (gpd) and phosphoglycerate mutase (gpm) genes have been found to be particularly effective for the expression of heterologous genes in oleaginous yeast. The promoter regions of the invention, intron and enhancer have been shown to drive high-level expression of genes involved in the production of ω-3 and ω-6 fatty acids.
Owner:EI DU PONT DE NEMOURS & CO
Preparation method of gel containing stem cell exosomes for repairing skin wounds
InactiveCN111420117AImprove application securityHigh clinical safetyCulture processSkeletal/connective tissue cellsMesenchymal stem cellEngineering
The invention relates to a preparation method of a gel containing stem cell exosomes for repairing skin wounds. The method comprises the following steps: 1) primary extraction and culture of human umbilical cord mesenchymal stem cells: 1.1) primary extraction of human umbilical cord mesenchymal stem cells, 1.2) subculture, and 1.3) collection of the culture supernatant; 2) extraction of human umbilical cord mesenchymal stem cell exosomes: 2.1) primary centrifugation, 2.2) secondary centrifugation, 2.3) removal of organelles by centrifugation, 2.4) coarse extraction of exosomes, and 2.5) finalextraction of exosomes; 3) preparation of a gel material: 3.1) preparation of chitosan, 3.2) configuration of beta-glycerol phosphate (beta-GP), and 3.3) preparation of the gel material; and 4) gel loading of the exosomes. The gel containing stem cell exosomes can promote repair of skin wounds, shorten the healing time of wounds and reduce scar formation.
Owner:陕西朗泰生物科技有限公司
Reconstituted cell for isoprene and preparation method of reconstituted cell
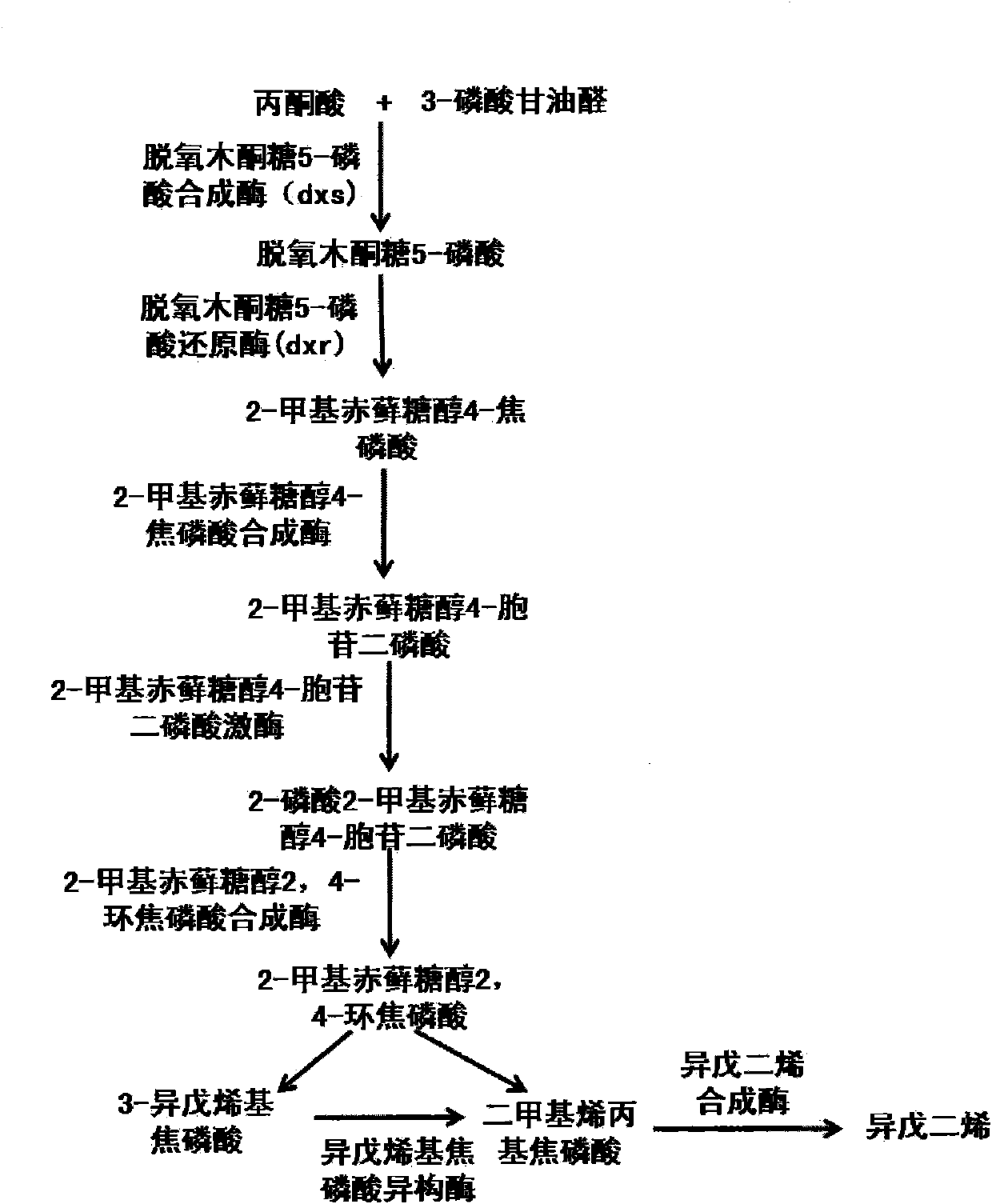
The invention provides a method for synthesizing isoprene from pyruvic acid and 3-phosphoglyceraldehyde, and a reconstituted cell. The pyruvic acid and the 3-phosphoglyceraldehyde are finally obtained from simple starting materials such as glucose.
Owner:QINGDAO INST OF BIOENERGY & BIOPROCESS TECH CHINESE ACADEMY OF SCI +2
Method for improving yield of arginine by mutation of Corynebacterium crenatum N-acetyl glutamic acid kinase
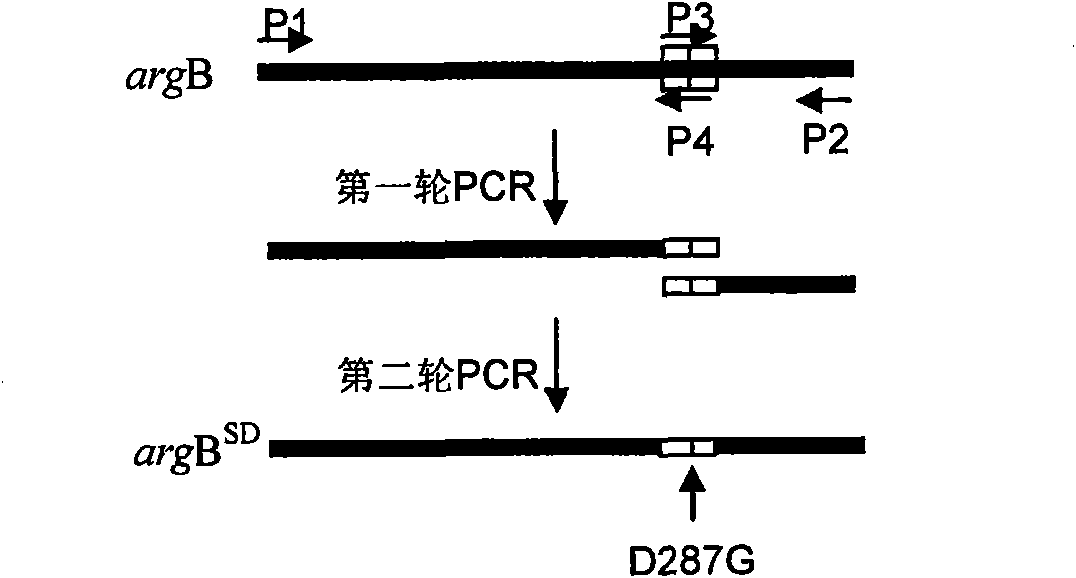
The invention relates to a method for improving the yield of arginine by the mutation of Corynebacterium crenatum N-acetyl glutamic acid kinase. L-arginine is one of semi-essential basic amino acids for a human body, and has various particular physiology and pharmacology functions. High-yield arginine mutant strain C. crenatum SYPA5-5 is compounded into the arginine through a circulating way, and N-acetyl glutamic acid kinase (NAGK) is a key enzyme in the compounding way and is subject to the feedback inhibition of the product arginine. By using an overlapping PCR (Polymerase Chain Reaction) technique, a GAT (glycerol phosphate acyl transferase) codon used for coding aspartic acid is used for substituting a GGA (General Gonadotropic activity) codon used for coding glycine at the site 287 in the coding NAGK albumen, and the NAGK which has high activity and arginine with obviously reduced by the feedback inhibition can be obtained after the mutation. An argBSD gene is brought into the Corynebacterium crenatum of the high-yield arginine through pJCl-tac, and the expression volume of the key enzyme is further improved. The final acid yield is increased to 36.3g / L from original 28g / L, and the yield of the L-arginine is increased by 29.7 percent.
Owner:JIANGNAN UNIV
Novel multivalent carrier vaccine for shrimp and application thereof
ActiveCN102895677AHigh temperature resistanceActual production application development valueAntibacterial agentsBacteriaWhite spot syndromeVector vaccine
The invention relates to a novel multivalent carrier vaccine for a shrimp. The vaccine is characterized by comprising a bacillus subtilis recombination strain obtained by a genetic engineering technique, the strain belongs to the category of bacillus subtilis HT5303 and is preserved in China Center for Type Culture Collection, and the preservation number is CCTCC NO.: M 2012395. When spores are formed by the bacillus subtilis vaccine strain, white spot syndrome virus VP281 protein can be demonstrated on the surfaces of the spores, and fusion protein of cell-penetrating peptides, white spot syndrome virus VP19 and vibrio harveyi 3-glyceraldehyde-phosphate dehydrogenase (GAPDH) can be secreted and expressed when vegetative cells are formed. Compared with other vaccines, the multivalent carrier vaccine has the advantages of cross protection and long protection period. Moreover, the vaccine can be added into feeds and has the advantages of resistance against high-temperature setting of feeds and broad prospects for commercial development.
Owner:马悦 +1
One-component dental adhesive compositions and method of use
A one-component self-etching self-priming dental adhesive composition is disclosed. The composition comprises glycerol phosphate di(meth)acrylate monomer, at least one mono-functional polymerizable monomer having just one ethylenically unsaturated group, at least one multi-functional polymerizable monomer having at least two ethylenically unsaturated groups, at least one aprotic solvent, at least one protic solvent, and at least one polymerization initiator.
Owner:THE KERR
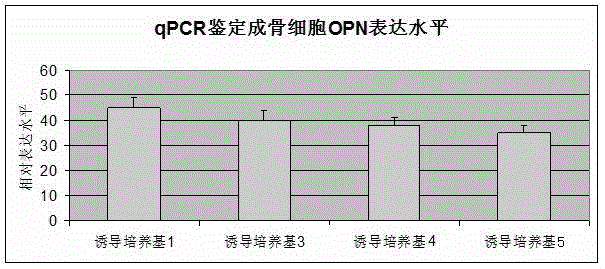
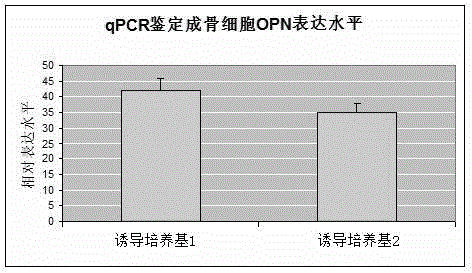
Osteogenic induction medium and osteogenic differentiation method
InactiveCN106434539AEffective osteogenic differentiationExcellent osteogenic differentiationCulture processSkeletal/connective tissue cellsDexamethasoneVitamin C
The invention relates to the field of the biotechnology, in particular to an osteogenic induction medium and an osteogenic differentiation method. The osteogenic induction medium is prepared from vitamin C, dexamethasone, beta-sodium glycerol-phosphate, bone morphogenetic protein-2, a vascular endothelial growth factor and a basic medium. Multiple inducing factors in the osteogenic induction medium are combined to act on GMSCs (gingival mesenchymal cells) and have a synergistic effect, the osteogenic differentiation of MSCs can be effectively induced, and the osteogenic differentiation effect is remarkably superior to that of a conventional induction method.
Owner:GUANGZHOU SALIAI STEMCELL SCI & TECH CO LTD
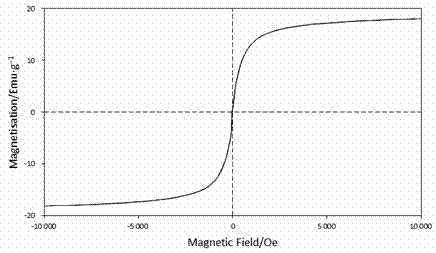
Method for preparing magnetic nano carrier immobilized aldolase with high substrate tolerance
InactiveCN102517276AIncreased substrate toleranceEasy to recycle and reuseOn/in inorganic carrierChemistryPrecipitation
The invention discloses a method for preparing magnetic nano carrier immobilized aldolase with high substrate tolerance. The method comprises the following steps of: 1) preparing super-paramagnetic Fe3O4 nano particles by co-precipitation of a mixture of ferrous and ferric iron salts, modifying the particles by using a silane coupling agent, and activating the outer surfaces of the modified particles by using glutaraldehyde to obtain surface activated magnetic nano particles; 2) stirring a purified and desalted aldolase buffer solution and a magnetic carrier at a low temperature, washing, performing freeze drying, and thus obtaining the magnetic nano carrier immobilized aldolase; and 3) catalyzing 2-deoxy-D-ribose-5-phosphoric acid by using the magnetic nano carrier immobilized aldolase as a catalyst to obtain 3-glyceraldehyde phosphate and acetaldehyde. In the reaction of catalyzing the 2-deoxy-D-ribose-5-phosphoric acid by using the immobilized aldolase, the tolerance of the acetaldehyde substrate is remarkably improved, and the recycling operation of enzyme is greatly simplified.
Owner:ZHEJIANG UNIV
Mesenchymal stem cell ossification osteogenic differentiation culture medium and preparation method thereof
InactiveCN104830758AImprove expression levelRich in nodulesSkeletal/connective tissue cellsMesenchymal stem cellGlutamine
The present invention provides a mesenchymal stem cell ossification osteogenic differentiation culture medium, and belongs to the technical field of stem cells. The mesenchymal stem cell ossification osteogenic differentiation culture medium comprises a DMEM / F12 culture medium, and further comprises FBS with a volume percentage of 5-50%, glutamine with a volume percentage of 0.5-5%, antibiotic with a volume percentage of 0.5-5%, 100-1000 [mu]M ascorbic acid, 5-50 mM glycerol phosphate, 5-50 nM dexamethasone, 5-50 [mu]M resveratrol, and 0.05-0.5 [mu]M puerarin. The mesenchymal stem cell ossification osteogenic differentiation culture medium of the present invention has advantages of high inducing efficiency and the like.
Owner:GUANGZHOU SALIAI STEMCELL SCI & TECH CO LTD
Multi-titer live vaccine as well as preparation method and application thereof
InactiveCN101659958AApparently multivalent immune protectionGood application prospectAntibacterial agentsBacterial antigen ingredientsPeptideSignal peptide
The invention provides a recombinant plasmid, a multi-titer live vaccine as well as preparation methods and the application thereof. The provided recombinant plasmid contains a fusion gene sequence ofsignal peptides of vibrio anguillarum metalloprotease and aeromonas hydrophila 3-glycerophosphate dehydrogenase; the preparation method of the recombinant plasmid comprises the following steps: (A) establishing signal peptide-3-glycerophosphate dehydrogenase fusion gene; (B) enzyme-cutting the fusion gene and a carrier; and (C) connecting the enzyme-cutting fusion gene and the enzyme-cutting carrier. The multi-titer live vaccine is prepared by converting the recombinant plasmid into vibrio anguillarum attenuated strains; and the preparation method of the multi-titer live vaccine comprises thefollowing steps: (A) establishing the recombinant plasmid containing signal peptides of vibrio anguillarum metalloprotease and aeromonas hydrophila 3-glycerophosphate dehydrogenase; and (B) converting the recombinant plasmid obtained in the step (A) into vibrio anguillarum attenuated strains. The multi-titer live vaccine is applied to prevent and treat fish diseases caused by vibrio anguillarum and aeromonas hydrophila. The attenuated vaccine provided by the invention has remarkable multi-titer immune protective efficiency, can be used as the live vaccine of vibrio anguillarum and aeromonas hydrophila and has favorable application prospect.
Owner:EAST CHINA UNIV OF SCI & TECH
Genetic engineering bacteria producing beta- carotene and construction method of genetic engineering bacteria
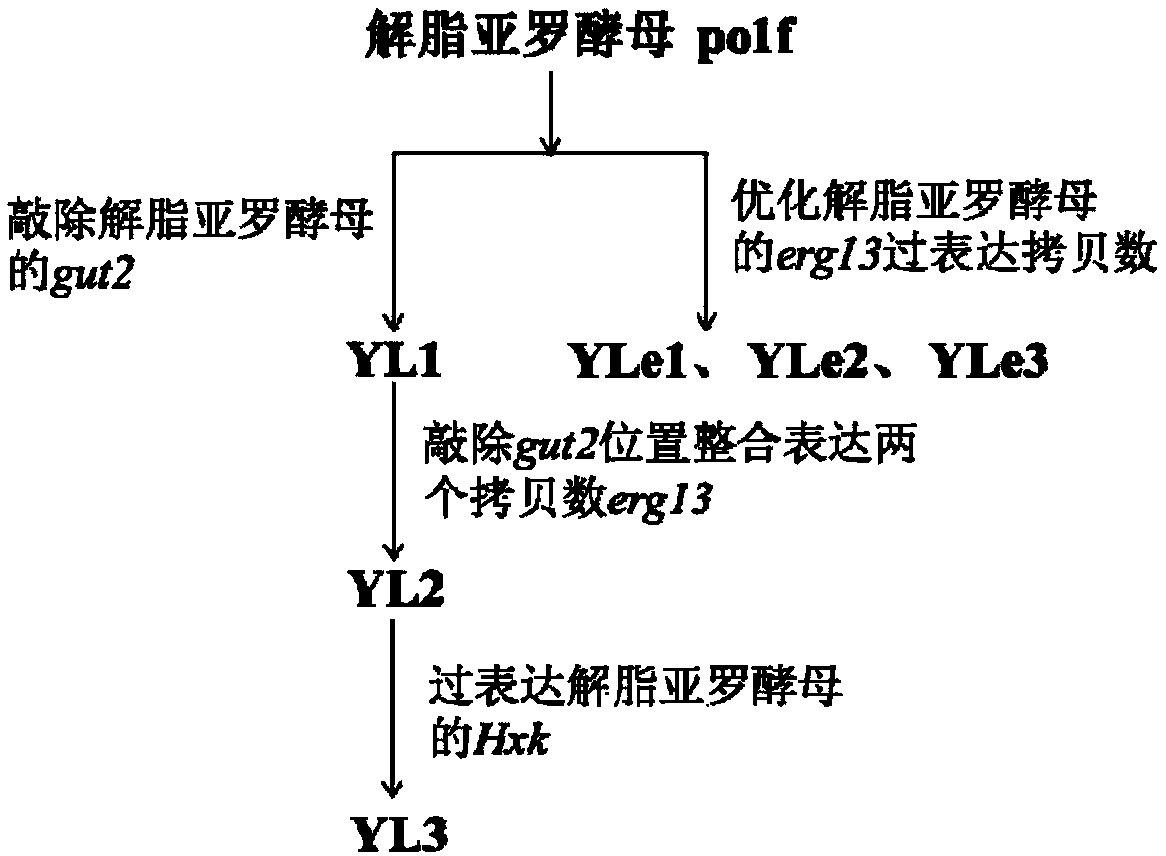
ActiveCN109609579AIncrease storage capacityIncrease productionFungiMicroorganism based processesBeta-CaroteneHexokinase
The invention discloses genetic engineering bacteria producing beta- carotene and a construction method of the genetic engineering bacteria. The construction method comprises the following steps of firstly knocking out alpha-glycerophosphate dehydrogenase gut2 genes in a glycolysis way in a beta-carotene synthetizing pathway in yarrowia lipolytica, then performing free expression on beta-hydroxyl-beta-methylglutaric acid erg13 genes having two endogenous copy numbers of the yarrowia lipolytica, and then performing integrant expression on the erg13 genes having two endogenous copy numbers of the yarrowia lipolytica at the position where the gut2 genes are knocked out; and performing free expression on endogenous hexokinase Hxk genes of the yarrowia lipolytica, and screening positive transformants to obtain the engineering bacterial strains producing beta-carotene yarrowia lipolytica. The bacterial strains are subjected to fermentation, culturing, extraction and separation, the beta-carotene is purified, and the content of the beta-carotene can reach 26.6mg / g cell dry weight.
Owner:SHAANXI NORMAL UNIV
Higher plant cytosolic er-based glycerol-3-phosphate acyltransferase genes
InactiveUS20060206960A1Modifying lipid metabolismChange outputSugar derivativesTransferasesBiotechnologyHeterologous
Glycerol-3-phosphate acyltransferase is the initial enzyme of the glycerolipid biosynthetic pathway. Biochemical analyses indicated that the reaction mediated by glycerol-3-phosphate acyltransferase represents a potential rate-limiting step for the synthesis of phospholipids and storage neutralipid, triacylglycerol. The present invention relates to the cloning of genes encoding extraplastidic membrane-bound glycerol-3-phosphate acyltransferases. Heterologous expression of the genes, GPAT1, GPAT2, and GPAT3 in a yeast glycerol-3-phosphate acyltransferase mutant demonstrated that the encoded products could efficiently utilize glycerol-3-phosphate to mediate sn-1 stereo-specific fatty acid acylation. The invention encompasses the glycerol-3-phosphate acyltransferase peptides disclosed and fragments and homologues thereof, the corresponding gene sequences and fragments and homologues thereof, as well as the use of the peptide and gene sequences of the present invention for use in generating recombinant proteins, and transgenic plants with altered lipid metabolism. In this way, the present invention also encompasses the use of such recombinant peptides and transgenic plants for the production of lipid products for use, for example, in pharmaceutical and nutritional applications.
Owner:NAT RES COUNCIL OF CANADA
Composition for enhancing water solubility of curcumin and preparation method of composition
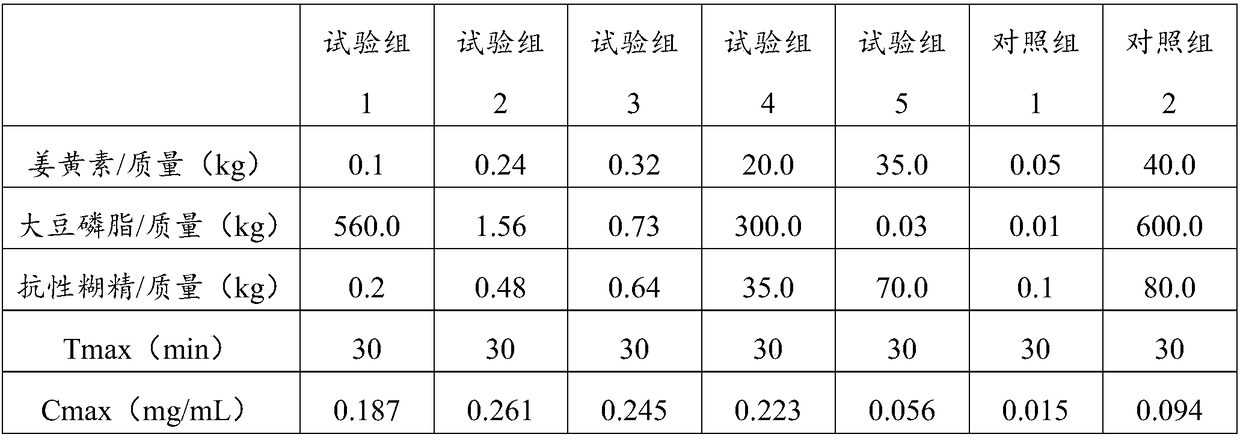
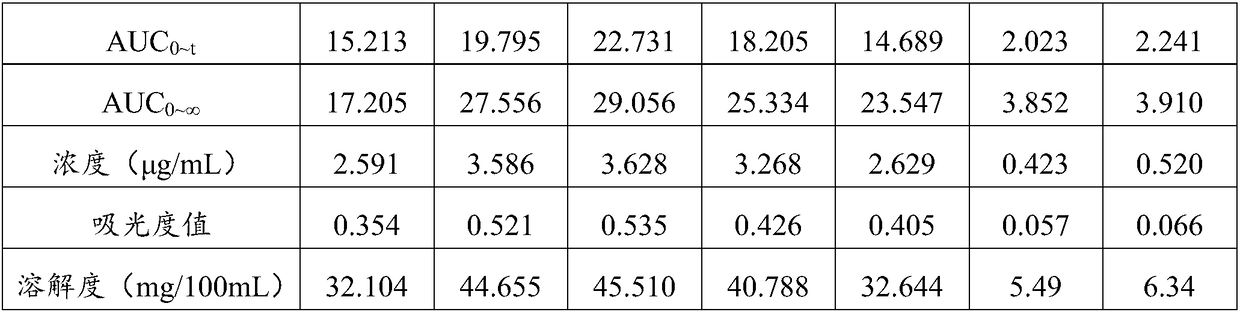
ActiveCN109481689ADoes not affect propertiesGood water solubilityAntipyreticAnalgesicsSolubilityWater soluble
The invention relates to a composition for enhancing the water solubility of curcumin and a preparation method of the composition and belongs to the technical field of foods and health-care products.The composition provided by the invention comprises the following components in parts by weight: 0.1 to 35.0 parts of the curcumin or a derivative thereof, 0.03 to 560.0 parts of phospholipid containing glycerol phosphate or the glycerol phosphate and 0.2 to 70.0 part of resistant dextrin. In the composition provided by the invention, the dissolution rate and the releasing rate of the composition,and the in-vivo transportation efficiency and the bioavailability of the curcumin are cooperatively enhanced through the glycerol phosphate and the resistant dextrin, and the intestinal absorption condition of the lipid-soluble curcumin is improved; and the types and forms, and a functional application range of a curcumin solid preparation are expanded under the condition that original propertiesand anti-oxidization performance of the curcumin are not influenced.
Owner:GUANGZHOU HANFANG PHARMA
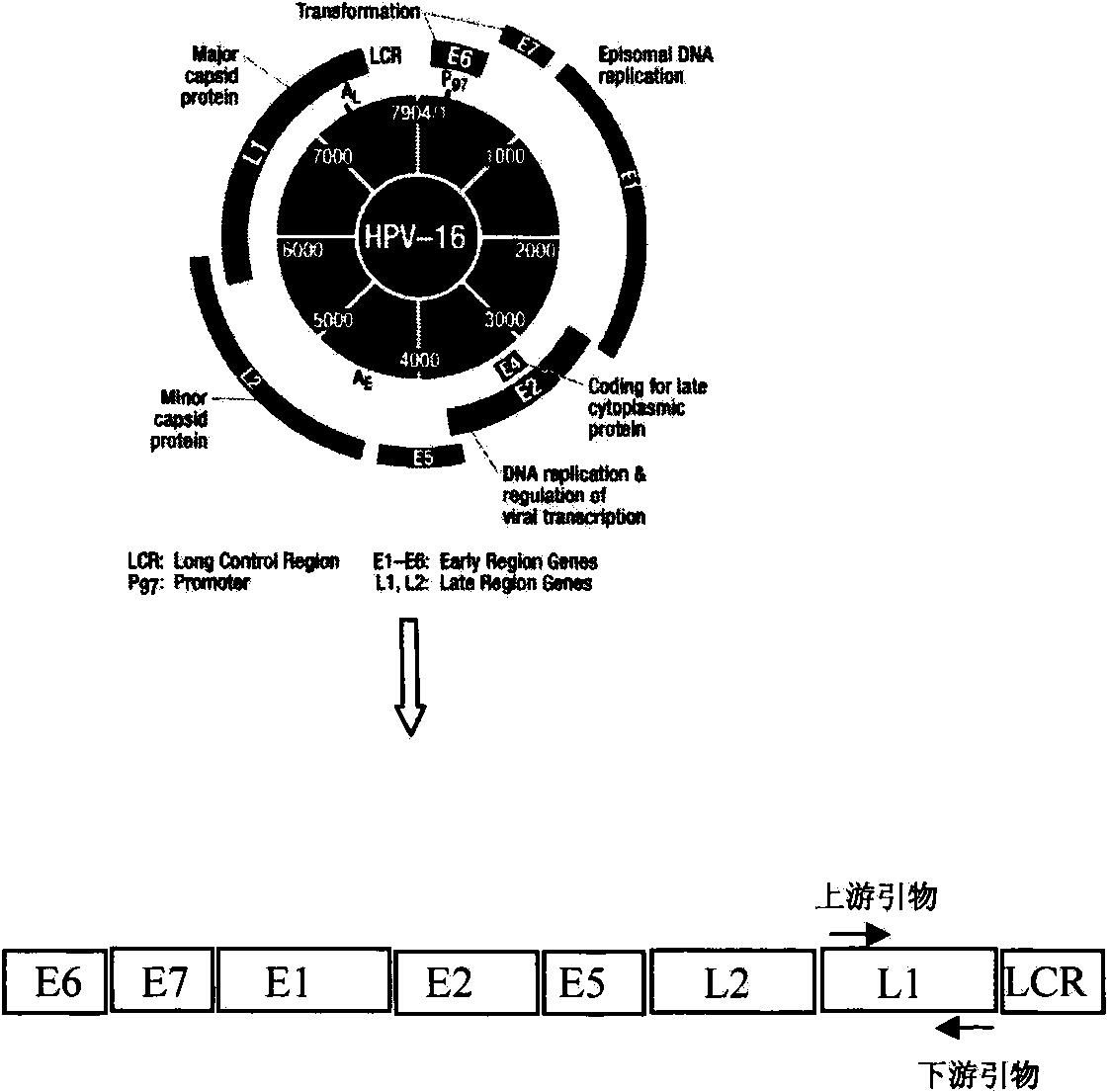
Broad-spectrum, efficient and economical PCR (Polymerase Chain Reaction) detection method for high-risk human papilloma virus
InactiveCN103409560AMicrobiological testing/measurementDeoxyuridine TriphosphatePolymerase chain reaction
The invention discloses a broad-spectrum, efficient and economical PCR (Polymerase Chain Reaction) detection method for high-risk human papilloma virus. The detection method comprises the following steps of (1) designing primers aiming at 12 high-risk HPV (human papilloma virus) subtype L1 genes, wherein the melt points of the all genes are 60 DEG C, and the gas chromatography (GC) percents are 50%, so that the primers can amplify virus gene sequences in the same PCR tube under the same temperature, so as to save time and money; (2) taking glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as a positive contrast, so as to avoid a false negative result; (3) avoiding a false positive result by utilizing an anti-pollution warm-start PCR system containing deoxyuridine triphosphate (dUTP) and uracil-N-glycosylase (UNG). According to the detection method, a PCR system is optimized based on the embodiment, and the specificity and the accuracy of a result are guaranteed, and according to the detection method, time saving and economy are realized, efficient and low-cost screening work is carried out on HPV viruses easily by a base hygiene department, and a purpose of preventing the cervical cancer is achieved.
Owner:潘晓静
Antibacterial silver ion compound, non-irritant silver ion antibacterial agent as well as preparation method and application of thereof
The invention belongs to the technical field of antibiosis, particularly relates to an antibacterial silver ion compound, which further relates to a non-irritant silver ion antibacterial agent as wellas a preparation method and an application thereof. The cationic ions in the antibacterial silver ion compound are coordination cations formed by silver ions and amino acid ligands. The amino acid ligand is selected from amino acid and / or polypeptide, and the anion is selected from one or more of acetate radical, hydrogen phosphate radical, dihydrogen phosphate radical, glycerol hydrogen phosphate radical, glycerol phosphate radical, lactate radical, glycolate radical, tartrate radical, citrate radical and malate radical. The antibacterial silver ion compound disclosed by the invention has good stability and compatibility, does not generate decomposition discoloration after being placed for a long time, does not have unpleasant odor, has good salt tolerance and is non-irritant. The antibacterial agents such as antibacterial liquid, antibacterial gel and the like prepared by taking the antibacterial silver ion compound as an active ingredient have better antibacterial property, are non-irritant and can be in direct contact with a human body.
Owner:洛阳冠银生物科技有限公司
Triglyceride determination kit and determination method thereof
ActiveCN108949903AImprove measurement accuracyImprove stabilityMicrobiological testing/measurementMethylanilineFlavin adenine dinucleotide
The invention provides a triglyceride determination kit. The kit comprises a liquid single reagent R1, the reagent R1 includes the following components with concentration: lipoprotein esterase, glycerol kinase, glycerol phosphate oxidase, peroxidase, piperazine-1,4-diethanesulfonic acid, NaOH, 4-aminoantipyrine, adenosine triphosphate, N-ethyl-N-(2-hydroxy-3-sulfopropyl)-3-methylaniline sodium orN-ethyl-N-(2-hydroxy-3-propyl)-3'5-dimethoxyaniline sodium salt, sodium glutamate, magnesium acetate, flavin adenine dinucleotide, polyethylene glycol, and a betaine solution. The kit belongs to the technical field of biological detection, and the triglyceride determination kit provided by the invention significantly enhances the anti-interference ability while improving the stability of the reagent, and has good measurement accuracy for triglyceride.
Owner:广州市伊川生物科技有限公司
Collagen polypeptide skin care mask and preparation method thereof
InactiveCN108434088AWell moisturizedGood effect of repairing the faceCosmetic preparationsToilet preparationsBetainePhosphoric acid
The invention discloses a collagen polypeptide skin care mask and a preparation method thereof. The mask comprises collagen polypeptide skin care mask cream and a mask carrier, wherein the mask creamis prepared from the following components by mass: 20-40 parts of butanediol, 10-15 parts of glycerol, 1-3 parts of betaine, 0.2-1 part of lactobacillus fermentation product, 0.2-1 part of panthenol,0.2-1.2 parts of sodium hyaluronate, 0.1-1 part of tocopherol, 2-5 parts of collagen, 0.3-2 parts of bishydroxymethyl imidazolidinyl urea, 0.3-0.8 part of allantoin, 0.1-1.2 parts of carbomer, 0.2-1 part of triethanolamine, 0.5-1 part of methylparaben, 1-5 parts of sclerotium gum, 0.1-1 part of glycerol phosphate inositol choline salt, 1-1.5 parts of oligopeptide-1, 1-1.5 parts of oligopeptide-2,0.4-1.5 parts of oligopeptide-5, 0.8-2 parts of EDTA disodium, 0.4-1 part of PEG-40 hydrogenated castor oil, 0.1-0.5 part of essence and 50-60 parts of water. The preparation method is used for preparing the collagen polypeptide skin care mask.
Owner:海南鳄珍鳄鱼产业科技有限公司
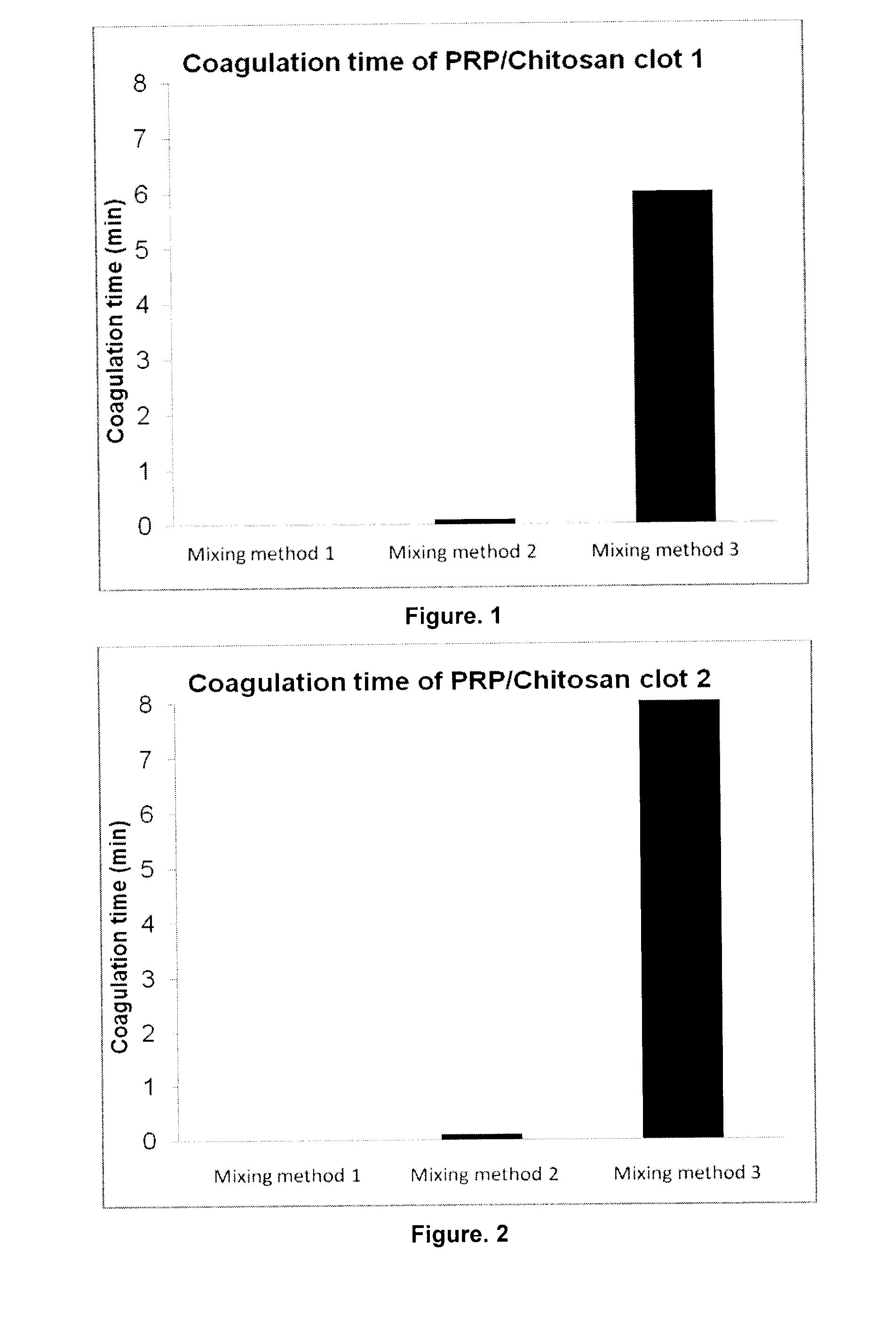
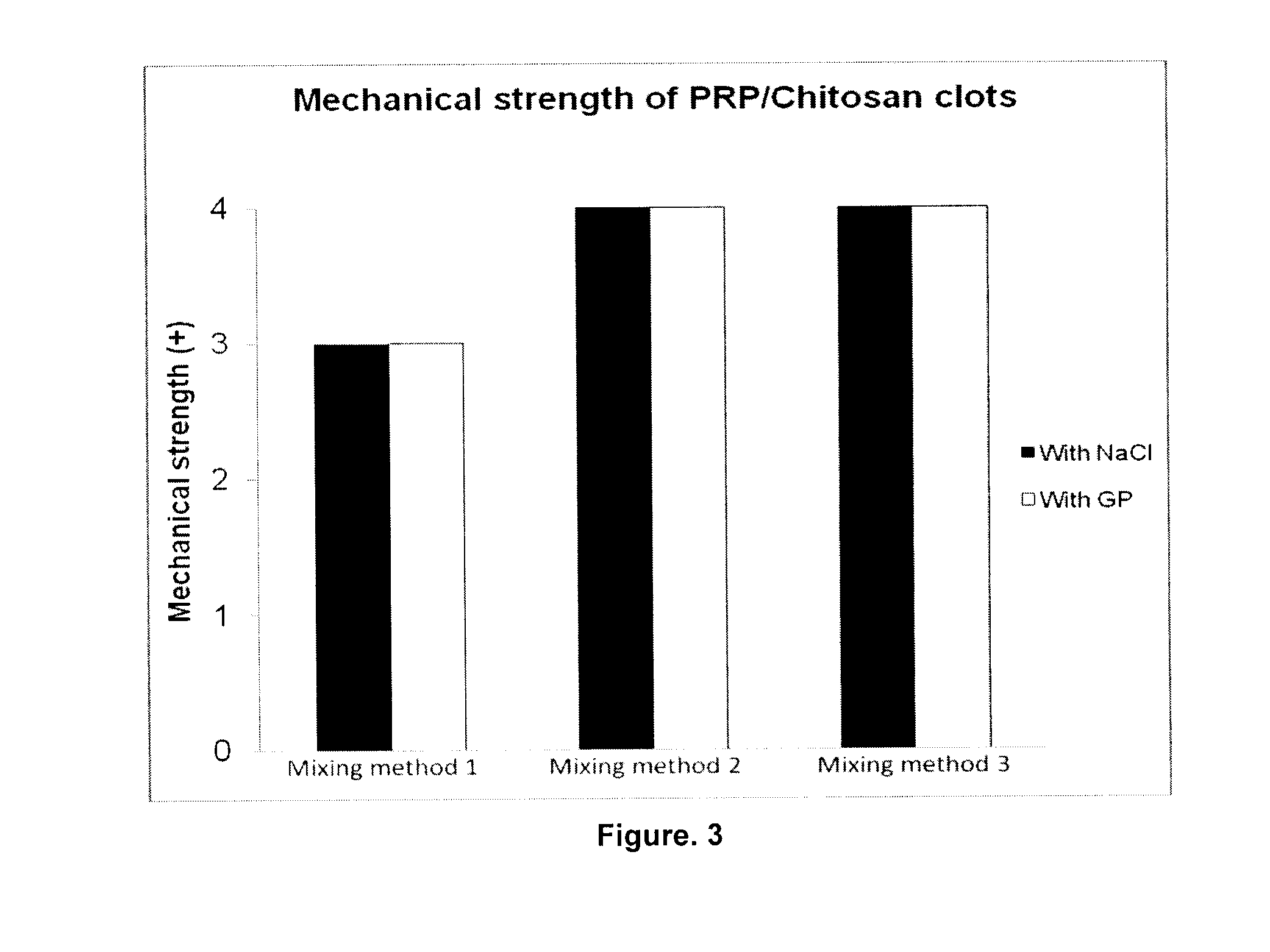
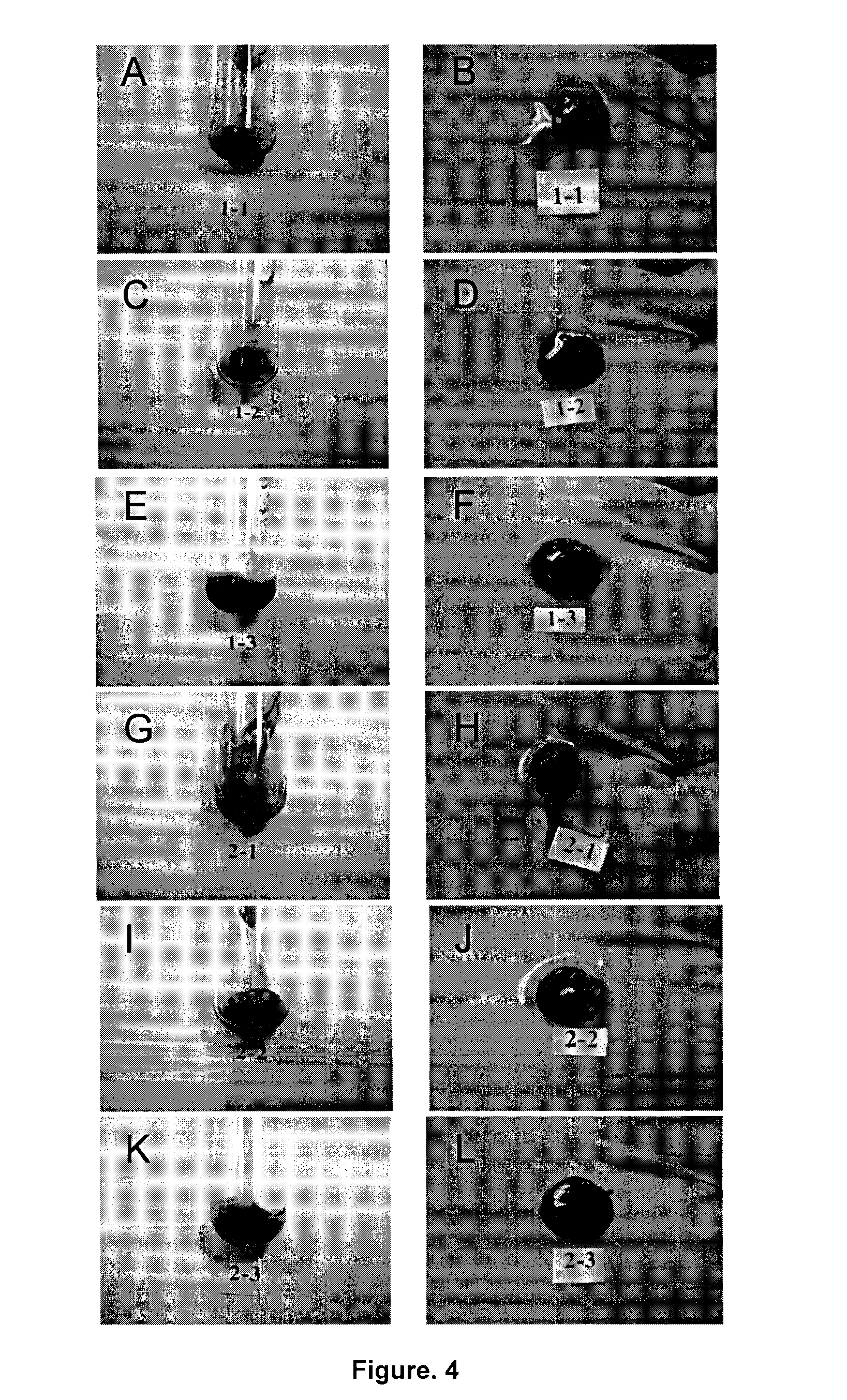
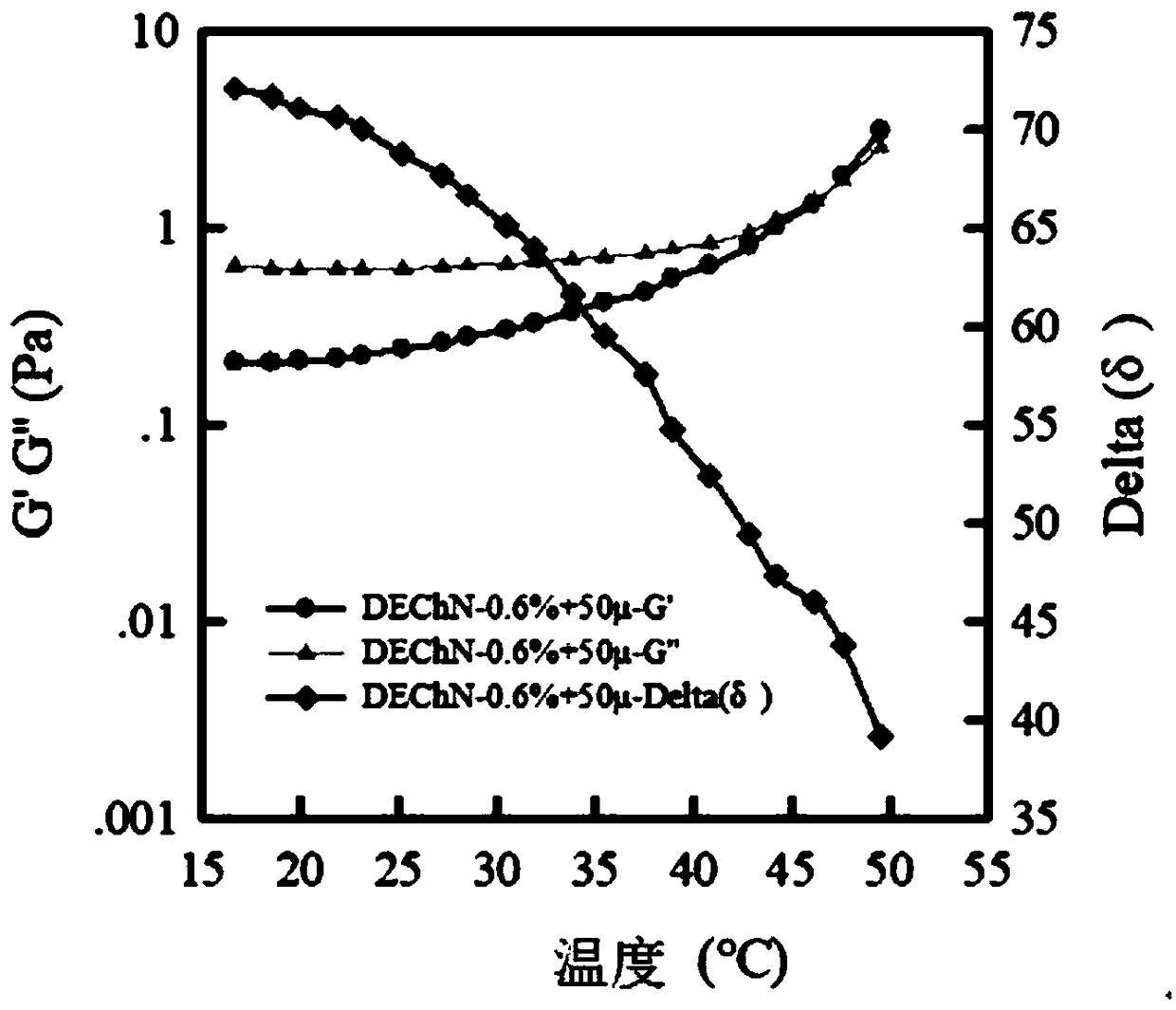
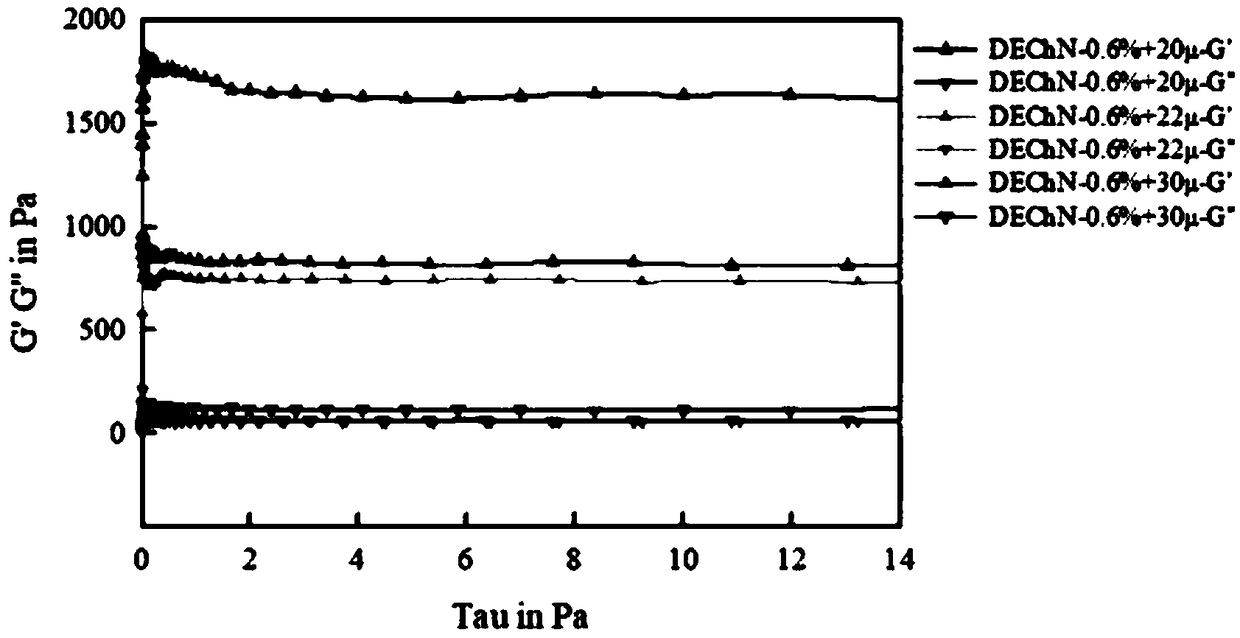
Soluble physiological chitosan formulations combined with platelet-rich plasma (PRP) for tissue repair
ActiveUS20130004474A1Promote cell proliferationBiocideOrganic active ingredientsTissue repairThrombin activity
Owner:ORTHO REGENERATIVE TECH
Temperature/pH double-response type chitin nano fiber hydrogel and preparation method thereof
ActiveCN109265758AIncrease temperatureExcellent pH sensitivityPharmaceutical non-active ingredientsFiberBiocompatibility Testing
The invention discloses temperature / pH double-response type hydrogel and a preparation method thereof. The method comprises the following steps: preparing partial chitosan; preparing a chitin nano fiber dispersion; slowly dropwise adding and mixing a water phase, an oil phase and a surfactant according to a proportion, and continuously stirring for 2-72 hours to obtain a clear transparent oil-in-water microemulsion; uniformly mixing the chitin nano fiber dispersion, the microemulsion, beta-phosphoglycerol and a biological complex factor according to a proportion, and performing alkaline coagulating bath to obtain the temperature / pH double-response type hydrogel. In the invention, the temperature / pH double-response type hydrogel is prepared by compounding the beta-GP and the biological complex factor with the chitin nano fiber, and the preparation method is simple and has strong operability. The temperature / pH double-response type hydrogel has good mechanical property, biocompatibility,temperature responsiveness, pH responsiveness and slow release property, and can be applied to the medical fields such as slow release of drugs, and thus has a good application prospect.
Owner:NANJING FORESTRY UNIV
Preparation method of temperature-sensitive chitosan hydrogel cell factor composite stent
InactiveCN108404207AImprove featuresEasy to makePharmaceutical delivery mechanismTissue regenerationDisodium glycerophosphateInsertion stent
The invention relates to the field of biological materials, and provides a preparation method of a temperature-sensitive chitosan hydrogel cell factor composite stent. According to the technical scheme, the preparation method of the temperature-sensitive chitosan hydrogel cell factor composite stent comprises the following steps: 1) mixing chitosan with gelatin at a mass ratio of 2.5: 0.8-1.2; 2)dissolving a mixture obtained from step 1) in an acetic acid solution with the concentration being 0.1 M or an aqueous solution, and blending for 24 hours or above at the temperature of 4 DEG C; 3) dropwise adding a glycerol phosphate disodium salt solution with the concentration being 44.4% into a mixture obtained from step 2), and adding in an ultra-clean table at the temperature of 4 DEG C while stirring, wherein the mass ratio of glycerol phosphate disodium salt to the mixture is 1: 0.5-1.0; 4) dropwise adding cell factors in the mixture obtained from step 3), and adding in the ultra-cleantable at the temperature of 4 DEG C while stirring; and 5) maintaining the mixture obtained from step 4) at 4 DEG C in a liquid state, and solidifying into solid gel at 37 DEG C 10 minutes later. Thestent obtained by the method can slowly release cell factors.
Owner:ZHEJIANG UNIV
Soilless cultivation nutrient formula of peonies and application thereof
InactiveCN104945162ASynchronous absorptionIncrease resistanceAgriculture gas emission reductionCultivating equipmentsAdditive ingredientNutrient solution
The invention discloses a soilless cultivation nutrient formula of peonies. The soilless cultivation nutrient formula is composed of, by weight, 13-16.5 parts of diphenylamine, 11.5-12.5 parts of ammonium ferric sulfate, 2-20 parts of auxin, 4-5 parts of methionine, 0.4-0.8 part of barium sulfide, 0.1-0.3 part of barium vanadate, 0.1-0.3 part of iodine pentafluoride, 0.05-0.2 part of glycerol phosphate calcium and the balance water. The soilless cultivation nutrient formula is made by weighing and evenly mixing the ingredients according to the ratio. By the adoption of the nutrient liquid, the seepage force and diffusivity of the nutrient liquid are greatly enhanced in the breeding process, so that the seeding stage is greatly shortened, the emergence rate is increased, the quality of emergence is improved, and meanwhile the cost is lowered; the nutrient liquid is more suitable for high-altitude low-temperature seedling cultivation.
Owner:汤在英
Vector comprising glucose promoter, host bacterium and preparation method of recombinant bovine-derived trypsinase
InactiveCN104195165AAvoid the disadvantages of being flammable, explosive and harmful to healthReduce volumeFungiMicroorganism based processesPichia pastorisZymogen
The invention relates to a method for expressing a bovine-derived trypsinogen gene in Pichia pastoris by gene recombination. The recombinant bovine trypsinogen is expressed in Pichia pastoris by the novel promoter and secreted into the culture medium; the zymogen is self-activated, or treated by enterokinase or trypsinase or the like to prepare the active bovine trypsinase; and the prepared active bovine trypsinase can be used for producing the recombinant human insulin and human insulin analogs. The method can avoid the defects in the constitutive promoter glyceraldehyde-3-phosphate dehydrogenase (GAP) promoter and the induction-type promoter alcohol oxidase (AOX1) promoter.
Owner:BEIJING AMBITION BIOTECH
Production process of transgenic rape as sinapic acid bioreactor
InactiveCN1436849AFermentationVector-based foreign material introductionF1 generationPhosphoric acid
The production process of transgenic rape as sinapic acid bioreactor includes the steps of: cloning phosphoenolpyruvate carboxyilase gene PEP segment and constituting it into Anti-PEG gene plant expression vector, introducing Anti-PEP gene into rape genome through gene conversion process to obtain "high-oil rape" with efficient oil synthesizing rape seed; preparing lytic phosphatidic acid acyltransferase (LPAAT) gene capable of connecting sinapic acid to the sn-2 site of 3-glycerol phosphate and connecting it to plant expression vector promoter to constitute LPAAT gene plant expression vector; introducing LPAAT gene into rape genome to obtain "high-sinapic acid rape" with efficient sinapic acid and 3-glycerol phosphate combining seed; crossbreeding "high-oil rape" and "high-sinapic acid rape" to obtain F1 generation rape plant with both "high-oil rape" characteristic and "high-sinapic acid rape" characteristic.
Owner:ZHEJIANG ACADEMY OF AGRICULTURE SCIENCES
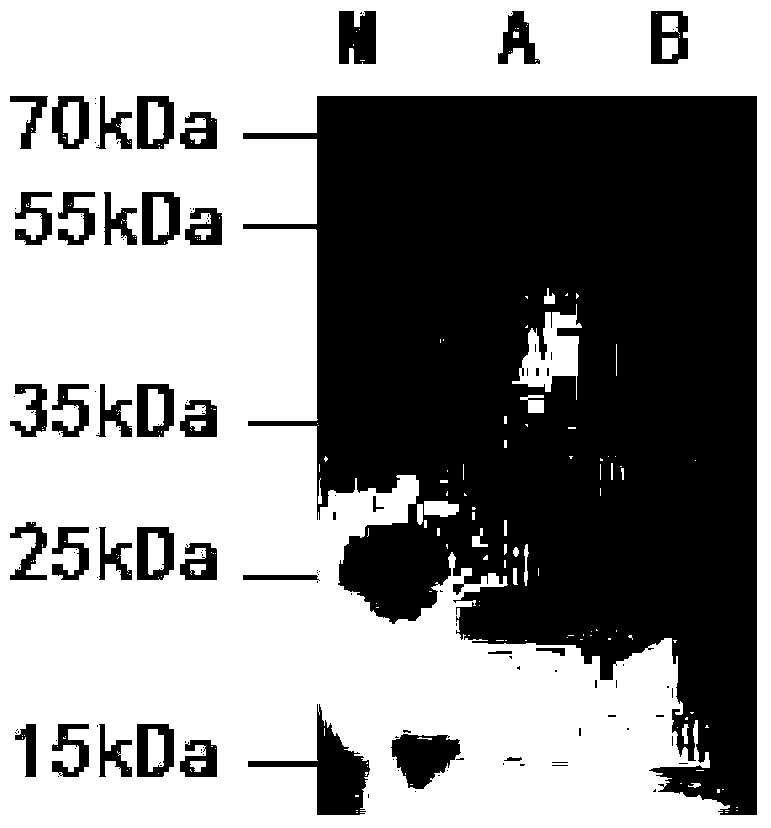
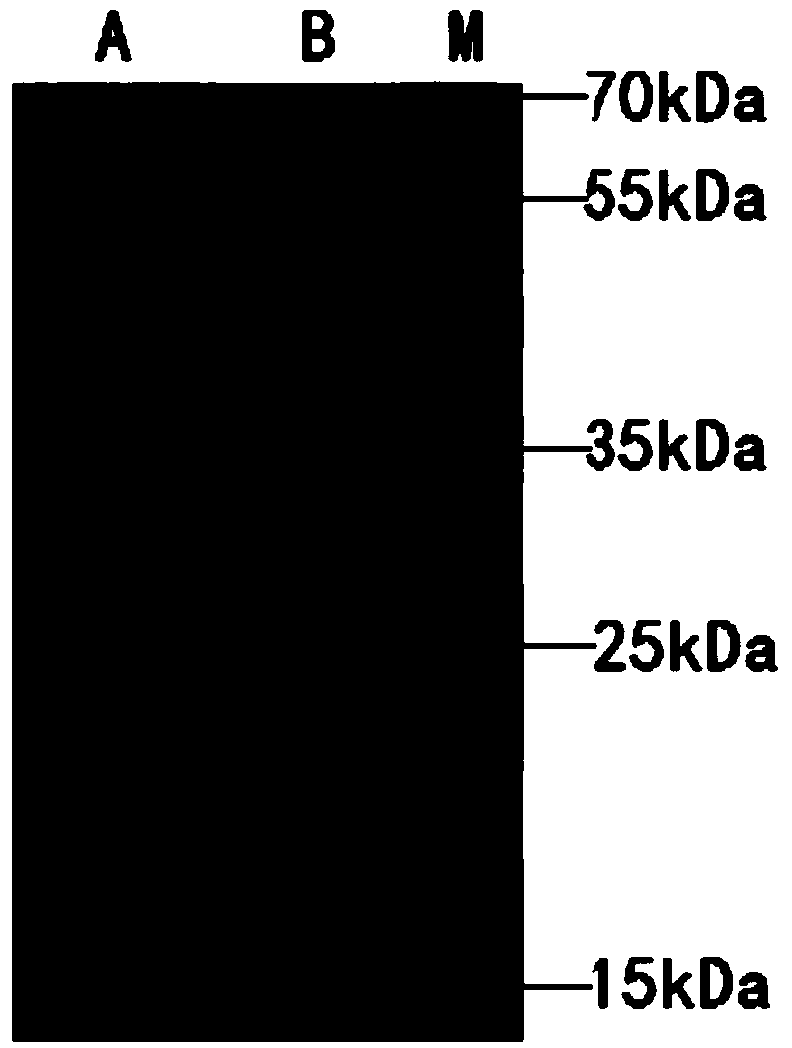
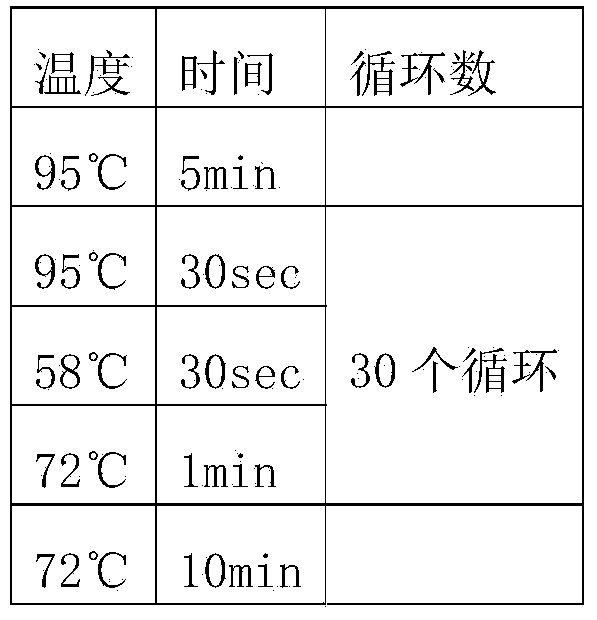
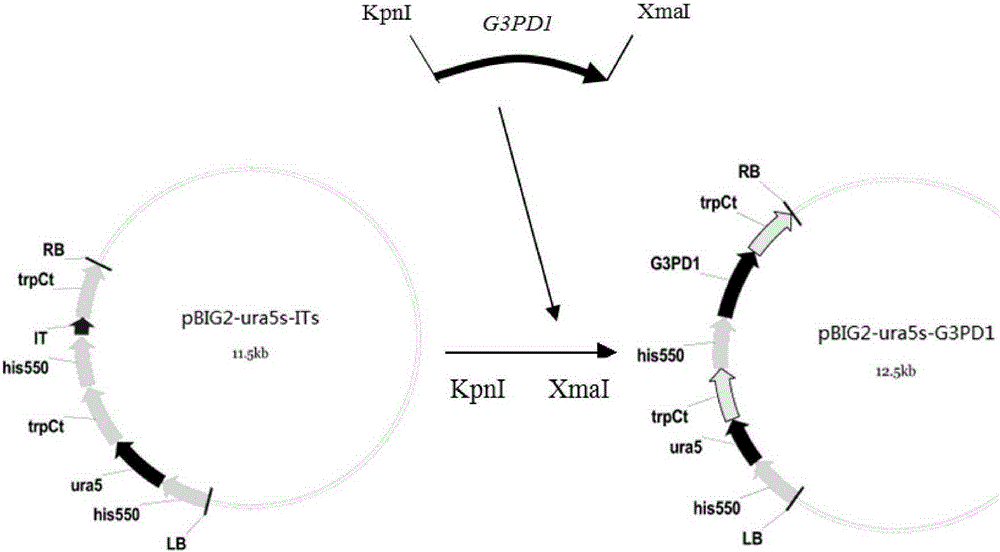
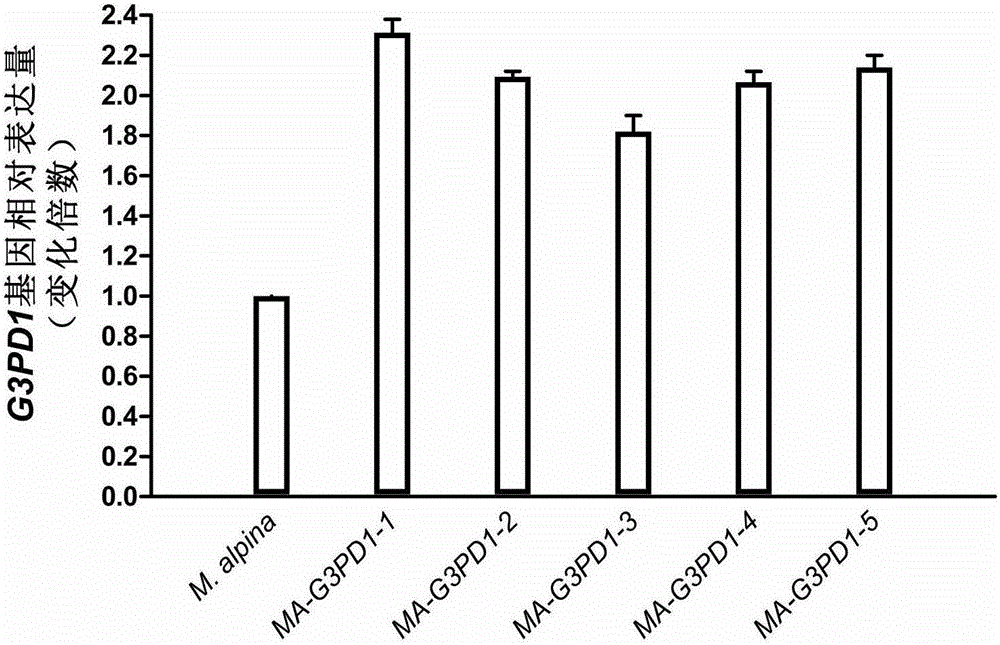
Mortierella alpine strain overexpressing 3-phosphoglycerol dehydrogenase gene(G3PD1), and construction method and application thereof
The invention relates to a recombinant Mortierella alpine strain GPD-1 overexpressing G3PD1 and a construction method. According to the invention, uracil-auxotrophic Mortierella alpine MAU1 is used as a material, Agrobacterium tumefaciens is used for mediation, and a G3PD1-overexpressing recombinant strain with obviously increased lipid content is obtained. Compared with wild Mortierella alpine, the total lipid content of the strain provided in the invention is increased by about 50%, and the transcription amount of G3PD1 is increased by about 2 times; so theoretical and application foundations are laid for subsequent industrial application.
Owner:JIANGNAN UNIV
D-3-phosphoglycerate-dehydrogenase as well as coding gene and construction method thereof
The invention relates to D-3-phosphoglycerate-dehydrogenase and a coding gene thereof. The amino acid sequence of the D-3-phosphoglycerate-dehydrogenase is shown as a sequence 1; the inhibition constant Ki of L-serine of the D-3-phosphoglycerate-dehydrogenase is greater than 250mM; and the sequence of the coding gene is shown as a sequence 2. The difference between the amino acid sequence of the D-3-phosphoglycerate-dehydrogenase (PGDH) provided by the invention and the amino acid sequence (sequence 3) of the escherichia coli wild type PGDH lies in that amino acid at 344th site is not histidine but lactamine and amino acid at 346th site is not asparagines but lactamine and the PGDH generated by mutation is not inhibited by the L-serine. In addition, the enzyme activity of a PGDH variant disclosed by the invention has no change compared with the wild type PGDH variant.
Owner:TIANJIN UNIVERSITY OF SCIENCE AND TECHNOLOGY
Nucleoside high-yielding strain as well as construction method and application thereof
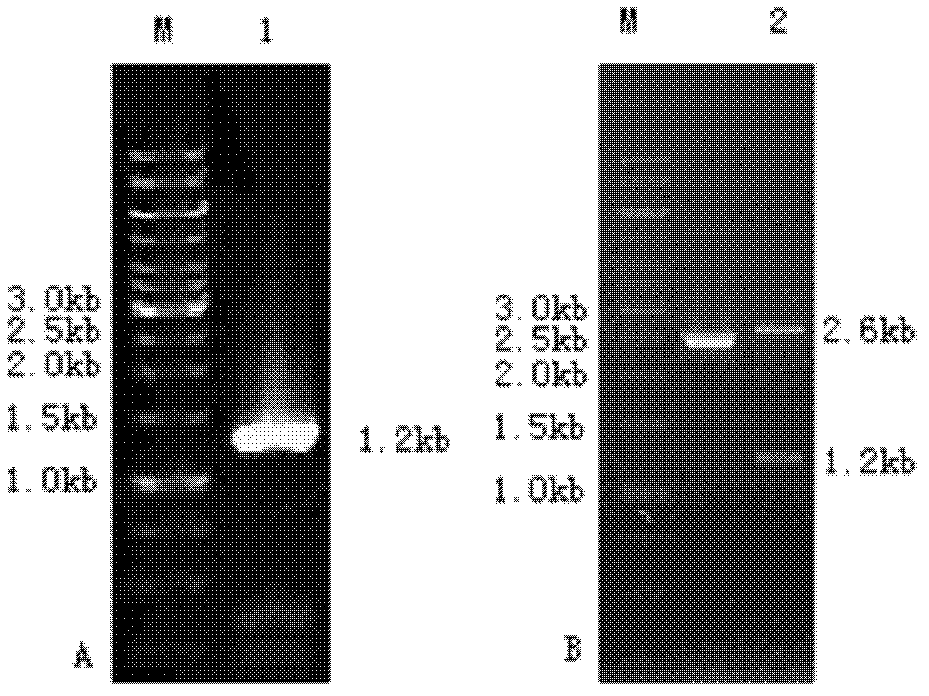
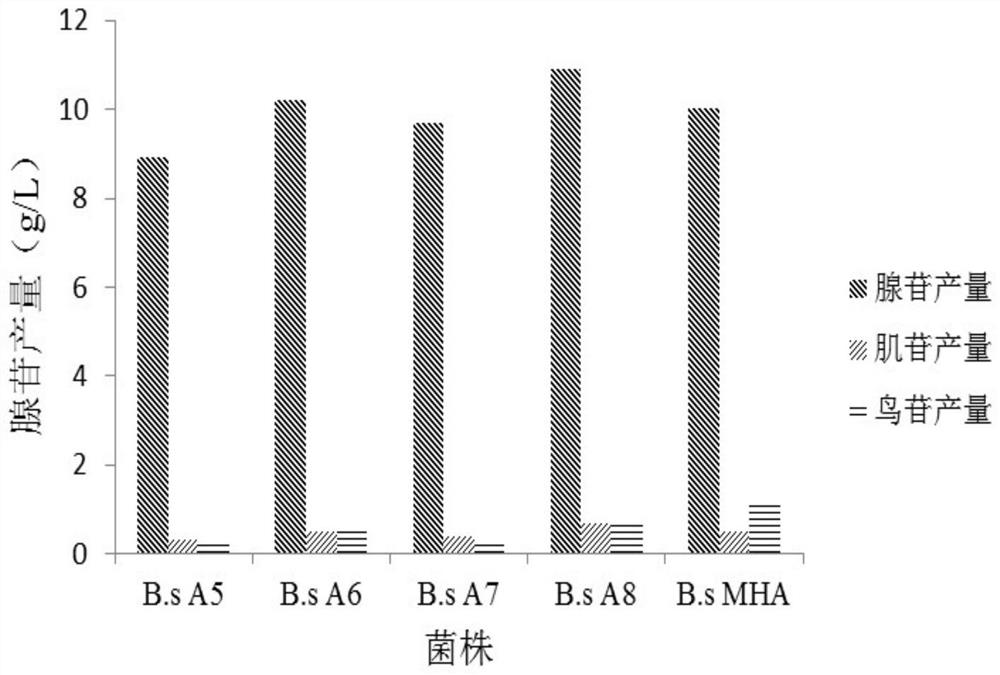
ActiveCN112126666AEfficient accumulationIncrease productionBacteriaTransferasesSerine hydroxymethyltransferaseBacilli
The invention provides a nucleoside high-yielding strain as well as a construction method and application thereof. The invention also provides a method for producing nucleoside by fermentation. The method comprises the following steps of (1) enhancing a serine hydroxymethyltransferase gene glyA, as shown in SEQ ID NO:2, of bacillus subtilis; and / or (2) weakening a 2,3-diphosphoglyceric acid independent phosphoglyceric acid mutant enzyme gene pgm of a coded NCBI reference sequence WP_003228330.1 on a chromosome of the bacillus subtilis; and (3) applying the strain obtained in the step (1) and / or the step (2) to fermentation production of the nucleoside. The engineered strain bacillus subtilis provided by the invention is the nucleoside high-yielding strain, can effectively accumulate the nucleoside, improves the yield of the nucleoside, and lays a foundation for industrial production of the nucleoside.
Owner:MEIHUA BIOTECH LANGFANG CO LTD
Reagent (kit) for measuring formaldehyde and method for measuring concentration of formaldehyde
InactiveCN101793788AFast measurementImprove accuracyMicrobiological testing/measurementColor/spectral properties measurementsAdditive ingredientPhosphoric acid
The invention relates to a reagent (kit) for measuring formaldehyde by using an enzyme-multiplied method, an enzyme colorimetric method and an enzyme coupling method as well as a method for measuring the concentration of the formaldehyde, and composition and ingredients of the reagent, belonging to the technical field of food / environmental test. The reagent (kit) comprises the main ingredients of buffer solution, coenzyme, adenosine triphosphoric acid, tetrahydrofolic acid, glyceric aldehyde-3-phosphoric acid, formaldehyde dehydrogenase, formic acid-dihydrofolic acid ligase, glyceric aldehyde-3-glycerol phosphate dehydrogenase and stabilizing agent. The concentration of the formaldehyde is measured by mixing a sample and the reagent according to a certain volume ratio to carry out a series of enzymatic reaction, placing the reactant under an ultraviolet / visible light analyzer and detecting the ascending degree of absorbance at a dominant wavelength of 340nm.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
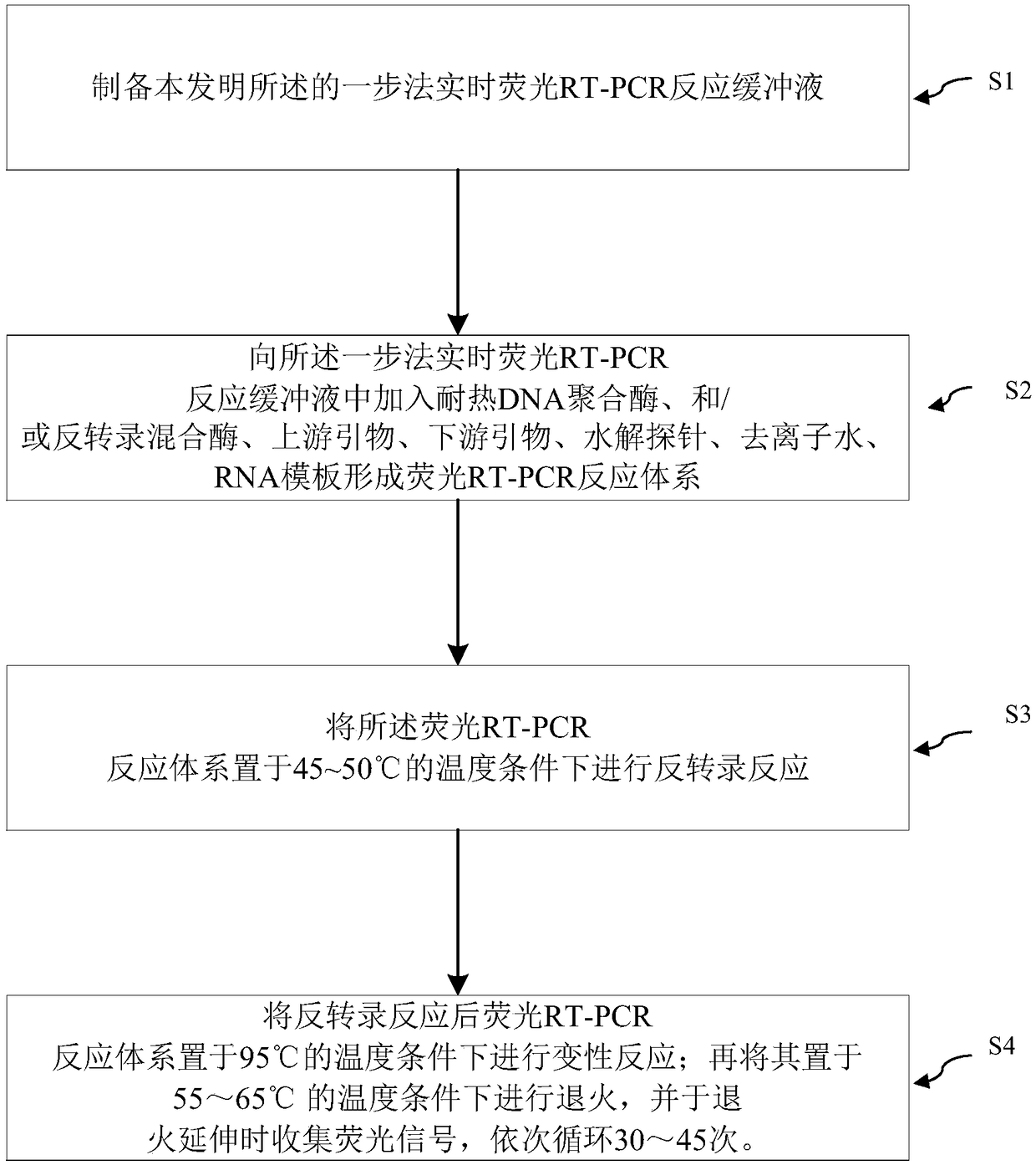
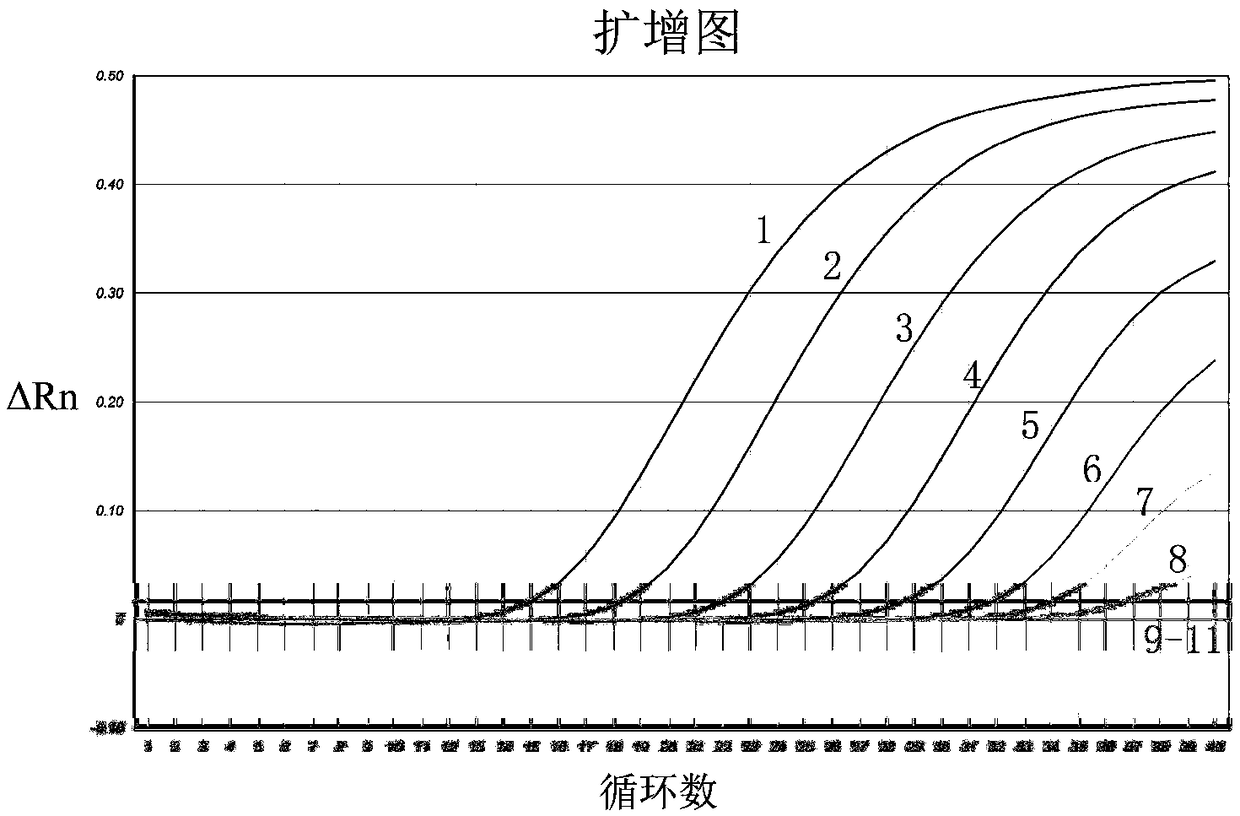
One-step real-time fluorescent RT-PCR reaction buffer and reaction system and PCR method thereof
ActiveCN108998506AImprove reaction efficiencyGood stabilityMicrobiological testing/measurementAmmonium sulfateDeoxyribonucleoside triphosphate
The invention relates to a one-step real-time fluorescent RT-PCR reaction buffer and a reaction system and a PCR method thereof. The one-step real-time fluorescent RT-PCR reaction buffer comprises thefollowing raw materials: a trishydroxymethyl aminomethane-hydrochloric acid buffer, potassium chloride, magnesium chloride, ammonium sulfate, dimethyl sulfoxide, deoxyribonucleoside triphosphate, glycerin, bovine serum albumin, Tween 20, sodium trinitride, a Rox reference dye and deionized water. The one-step real-time fluorescent RT-PCR reaction buffer increases NH4<+> and N3<->, improves the PCR reaction efficiency, is applicable to high-efficiency amplification and sensitive detection in a PCR technology, and not only has the advantages of good stability, a good fluorescence effect and high sensitivity. Through use of the one-step real-time fluorescent RT-PCR reaction buffer for preparing the fluorescent RT-PCR reaction system, the real-time fluorescent PCR method has the advantages ofa reliable, accurate and sensitive result, simple operation, time saving, labor saving, reduction in the detection cost, improvement on the detection efficiency and the like.
Owner:SHENZHEN ZIJIAN BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com