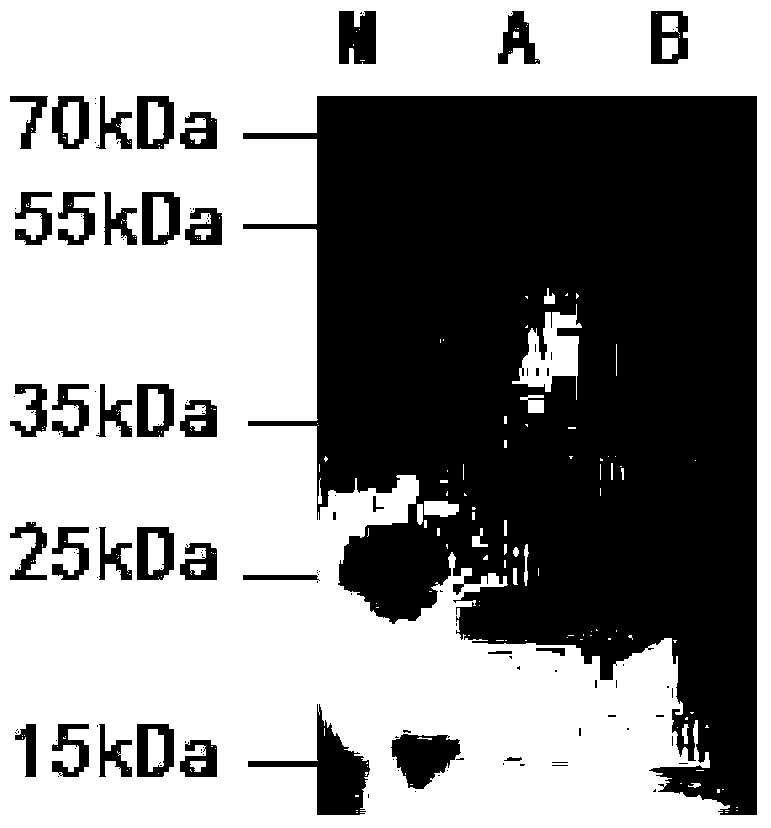
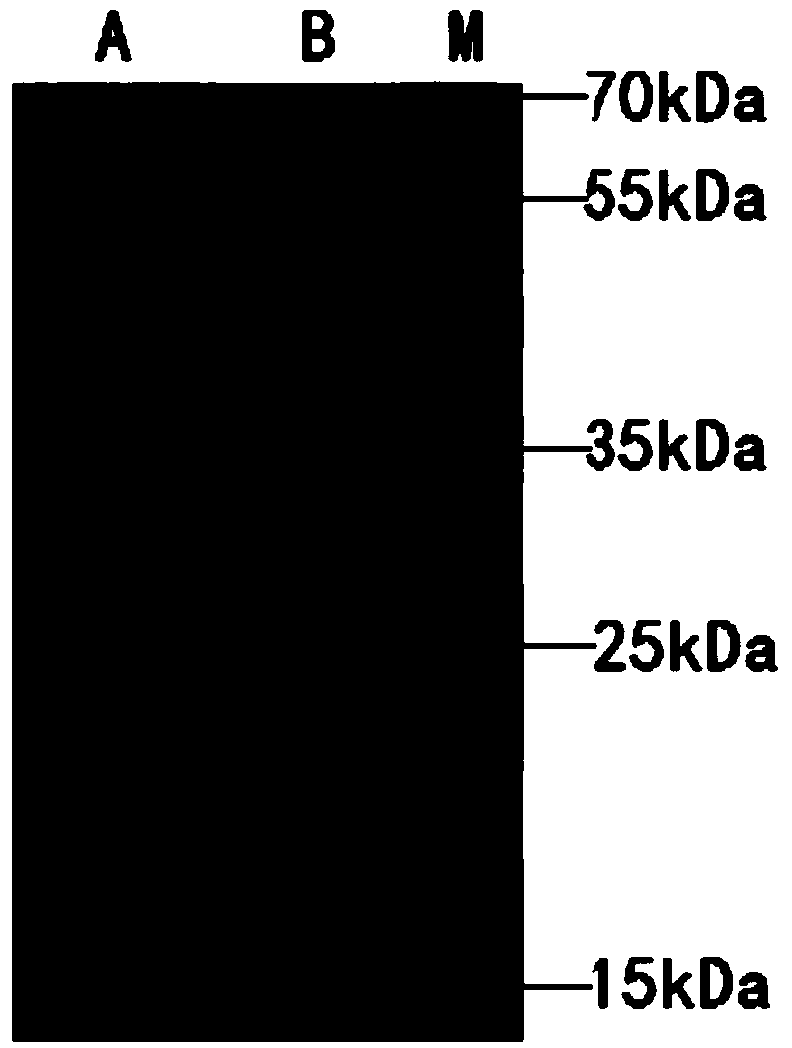
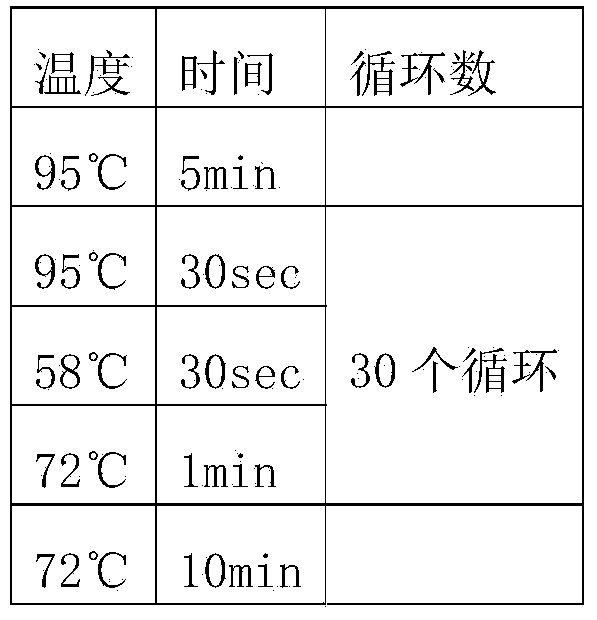
Vector comprising glucose promoter, host bacterium and preparation method of recombinant bovine-derived trypsinase
A technology of glucose promoter and bovine trypsin is applied in the preparation field of a carrier containing a glucose promoter, host bacteria and recombinant bovine trypsin, which can solve the problems such as the inability to completely remove other proteins, and achieves small size, high-efficiency expression, purifying simple effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1 Extraction of Pichia pastoris genome and amplification of PG6 promoter
[0056] 1. Streak PichiapastorisX33 onto YPD solid medium, culture at 30°C for 2 days, pick a single colony and inoculate it into YPD liquid medium, and after culturing for 1 day, extract the genome according to the instructions of Tiangen Yeast Genome Kit.
[0057] 2. Use the genome extracted in step 1 as a template, and use the designed two primers as upstream and downstream primers to amplify the PG6 promoter.
[0058] PG6-F: CGAAGATCTAGACCAGCAGTTTAACTACG (the underline is BglII restriction enzyme)
[0059] PG6-R: CCTGCCTTCGAACTTTTCTTTGGGCAAGGAAA (the underline is BstBI restriction enzyme)
[0060] PCR reaction system:
[0061] components
Volume (uL)
10×TransTaqHiFiBufferI
5
dNTPs (2.5mM)
4
Upstream primer PG6-F (10uM)
2
Downstream primer PG6-R (10uM)
2
DNA polymerase TaqHiFi (5U / uL)
0.5
PichiaX33 genome
1 ...
Embodiment 2
[0066] Embodiment 2 Transformation of pPICZαB vector
[0067] Digest pPICZαB with BglII and BstBI, recover a fragment of about 2700 bp, connect the 2700 fragment with the PG6 product in Example 1 with BglII and BstBI, and then use T4DNAligase, named: ZαB-PG6, the connection system is as follows:
[0068] components
[0069] The ligation product was transformed into TOP10, the positive clones were screened by colony PCR, and the positive clones were identified by plasmid digestion and sent to Jinweizhi for sequencing.
Embodiment 3
[0070] Example 3 Construction of bovine trypsin (BovineTrypsin, BT) former expression plasmid using ZαB-PG6
[0071] The nucleotide sequence encoding bovine trypsinogen was chemically synthesized according to the codon preference of Pichia pastoris, and the final nucleotide sequence was the nucleotide sequence shown in SEQ NO:2.
[0072] The reaction primer sequence is:
[0073] Upstream primer ZαB-BT-F:
[0074] AAGCTCGAGAAAAGATTTCCAGTTGATGATGATGATAAGATT (the XhoI recognition site is underlined)
[0075] Downstream primer ZαB-BT-R:
[0076] ATATAGCGGCCGCGTTAGAAGCAATAGTTTGCTTAATCC (the underline is the NotI recognition site)
[0077] Use the above primers to amplify the BT sequence with XhoI and NotI restriction sites, purify the PCR product with an agarose gel recovery kit, and double-enzyme ZαB-PG6 and PCR product with XhoI and NotI, respectively. The fragments were ligated with T4DNAligase, named: ZαB-PG6-BT, and the ligation system was as follows:
[0078] co...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Enzyme activity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com