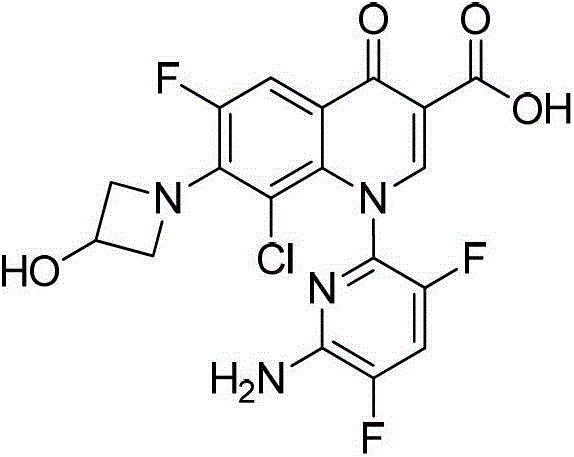
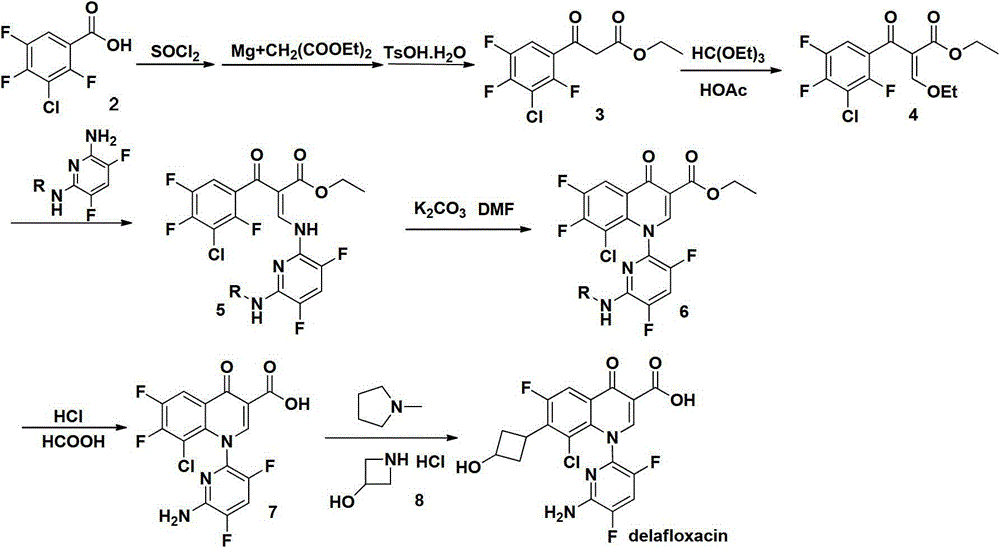
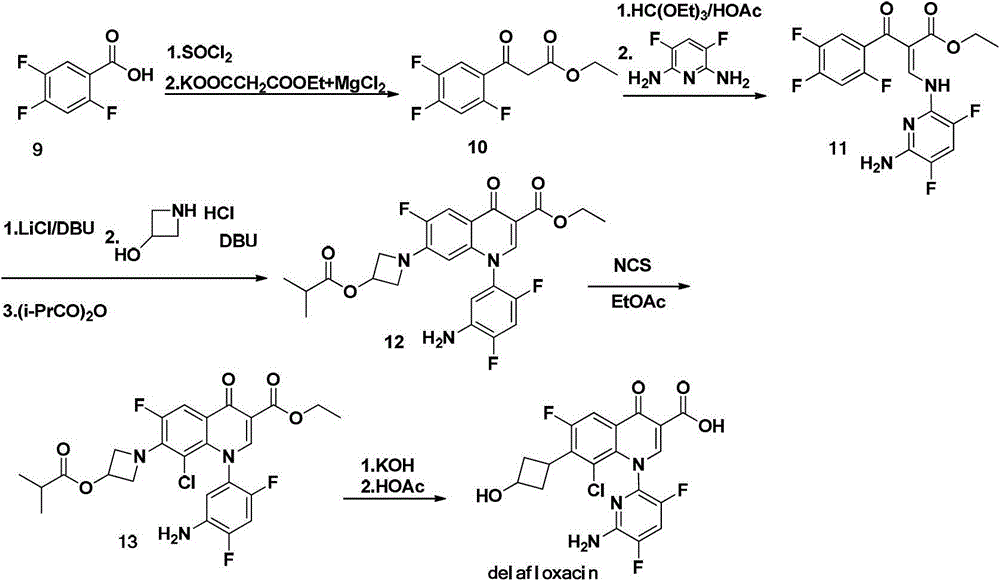
Intermediate of delafloxacin and preparation method thereof
The technology of a general formula compound and an alkyl group, which is applied to an intermediate of delafloxacin and its preparation field, can solve the problems of increasing hydroxyl protection steps, reducing yield and purity, and achieving comprehensive comparison of purity and yield, and reaction operation Simple, high-yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Preparation of the compound represented by formula 5 where R is ethyl:
[0039]
[0040] Add compound 3 (20g, 0.071mol), triethyl orthoformate (18.97mL, 0.11mol) and acetic anhydride (20.21mL, 0.21mol) into a three-necked flask, stir and heat to reflux for reaction (~139℃) for 3h, and reduce to At room temperature, the reaction solution was diluted with NMP-acetonitrile (50mL-50mL), and 1mL distilled water was added to prepare compound 4 directly into the next reaction without separation.
[0041] Add 2,6-diamino-3,5-difluoropyridine (11.38g, 0.078mol), NMP-acetonitrile (50mL-50mL) into a three-necked flask, stir to dissolve, add dropwise the previous reaction solution at room temperature, dropwise The reaction was stirred at room temperature for 1h. The reaction solution was added dropwise to 160 mL of distilled water to precipitate a bright yellow solid, filtered, and washed with acetonitrile-water (48 mL-24 mL) and water (50 mL) successively, and dried under vacuum at 60...
Embodiment 2
[0043] Preparation of the compound represented by formula 5 where R is ethyl:
[0044]
[0045] Add compound 3 (20g, 0.071mol), triethyl orthoformate (18.97mL, 0.11mol) and acetic anhydride (20.21mL, 0.21mol) into a three-necked flask, stir and heat to 100℃, reflux and react for 10h, then cool to room temperature. The solution was diluted with NMP (100 mL), and 1 mL of distilled water was added to prepare compound 4 directly into the next reaction without separation.
[0046] Add 2,6-diamino-3,5-difluoropyridine (11.38g, 0.078mol), acetonitrile (100mL) to a three-necked flask, stir to dissolve, at room temperature, add dropwise the reaction solution from the previous step, and stir the reaction at room temperature. 1h. The reaction liquid was added dropwise to 160 mL of distilled water to precipitate a bright yellow solid, which was filtered and dried to obtain 28.1 g of yellow powder with 98.1% HPLC. Melting point: 148-150℃1HNMR (400MHz, CDCl3) δ 1.13 (t, 3H), 4.25 (q, 2H), 4.66...
Embodiment 3
[0048] Preparation of the compound represented by formula 5 where R is ethyl:
[0049]
[0050] Add compound 3 (20g, 0.071mol), triethyl orthoformate (18.97mL, 0.11mol) and acetic anhydride (20.21mL, 0.21mol) into a three-necked flask, stir and heat to reflux for reaction (~139℃) for 3h, and reduce to At room temperature, the reaction solution was diluted with NMP (100 mL), and 1 mL of distilled water was added to prepare compound 4 directly into the next reaction without separation.
[0051] Add 2,6-diamino-3,5-difluoropyridine (11.38g, 0.078mol), NMP (100mL) to a three-necked flask, stir to dissolve, add dropwise the reaction solution from the previous step at room temperature, and stir the reaction at room temperature. 1.5h. The reaction liquid was added dropwise to 160 mL of distilled water, and a bright yellow solid was precipitated, which was dried to obtain 25.2 g of yellow powder with 98.0% HPLC. Melting point: 148-150℃1HNMR (400MHz, CDCl3) δ 1.13 (t, 3H), 4.25 (q, 2H), 4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com