Vaccine for prevention of toxoplasma infection as well as preparation method and application of vaccine
A technology for infection prevention and Toxoplasma gondii, which is applied in the fields of immunology and molecular biology, can solve the problems of high technical difficulty, weak anti-infection ability, formation of tissue cysts, etc., and achieve the effect of prolonging survival time and reducing the amount of worms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
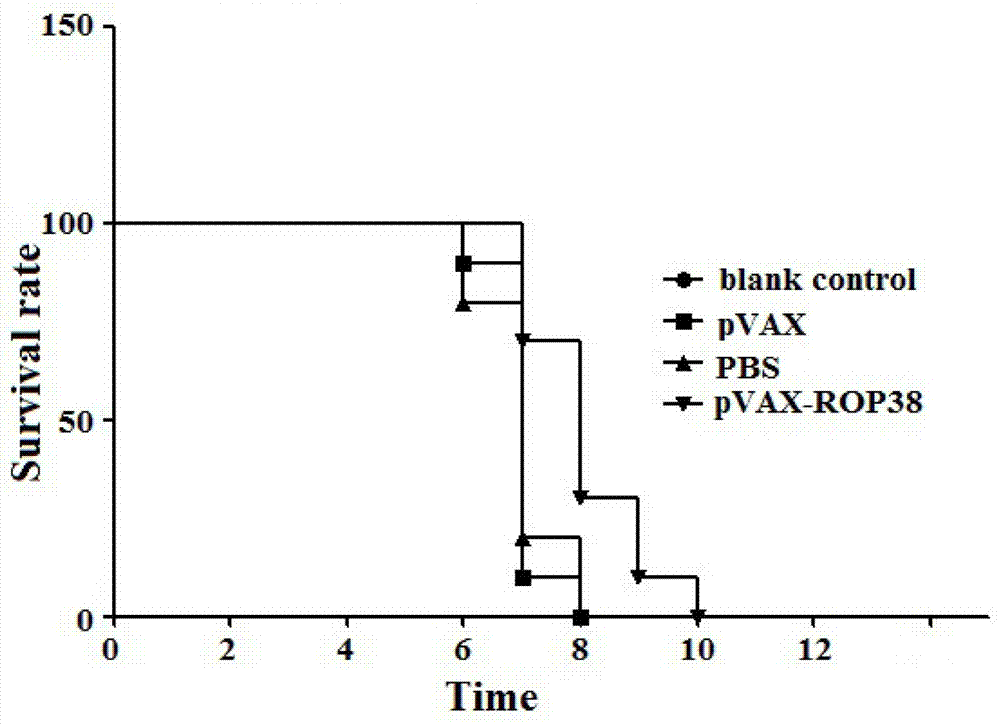
[0034] The following examples facilitate a better understanding of the present invention, but do not limit the present invention. The experimental methods in the following examples are conventional methods unless otherwise specified. The test materials used in the following examples, unless otherwise specified, were purchased from conventional biochemical reagent stores. Quantitative experiments in the following examples were all set up to repeat the experiments three times, and the results were averaged.
[0035] In the present invention, experimental materials are purchased from:
[0036] Female KM mice, about 6 weeks old, weighing 20±2g, were purchased from the Medical Experimental Center of Lanzhou University.
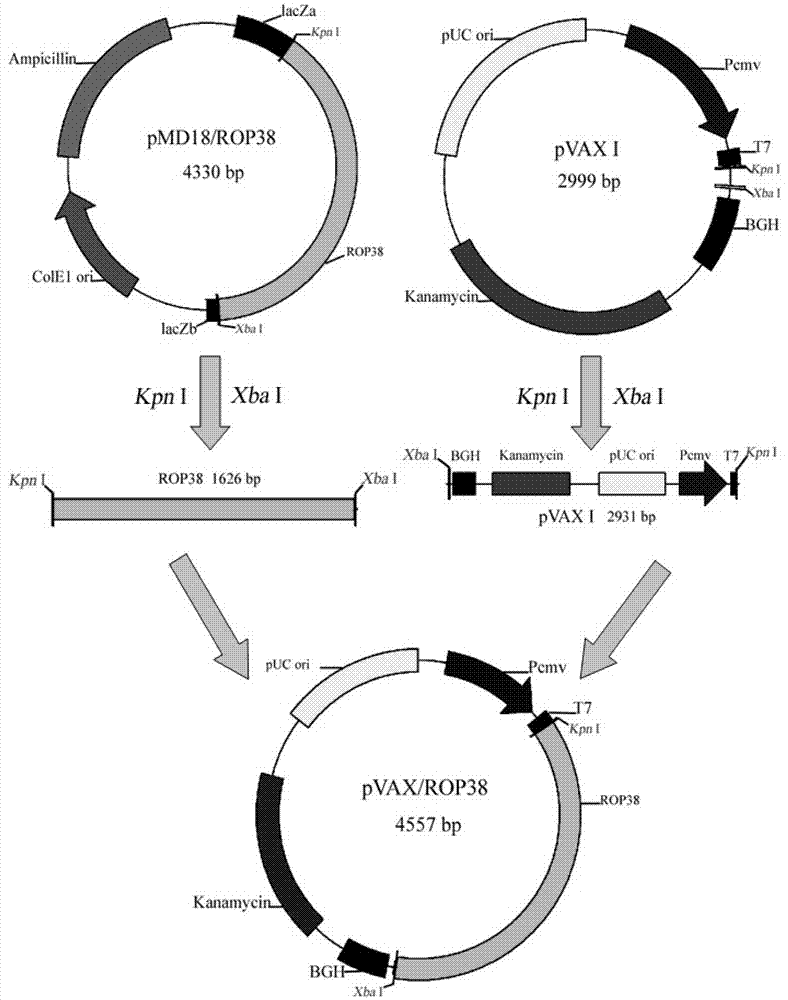
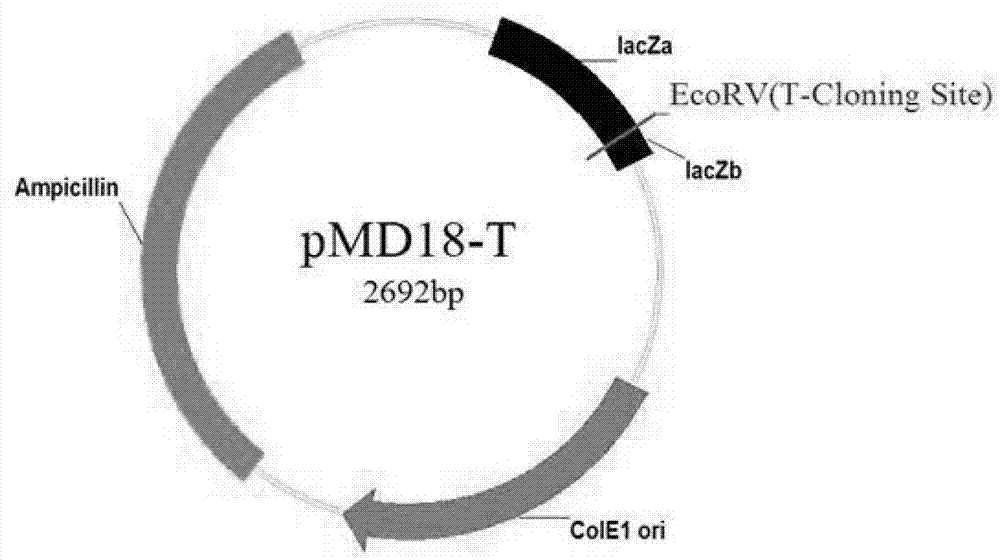
[0037] Host bacteria, Toxoplasma gondii RH, PRU strains, pVAX I eukaryotic expression vector and other materials were all preserved and provided by the Laboratory of Parasite Functional Genomics, Lanzhou Veterinary Research Institute, Chinese Academy of Agricultu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com