Patents
Literature
84 results about "Toxoplasma gondii Infections" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Toxoplasma gondii is a common parasitic organism frequently carried by domestic and feral cats that may cause infection in humans, known as toxoplasmosis.
Kit for detecting cysticercus cellulosae, swine trichinella and swine toxoplasmosis and application
ActiveCN102304578ASimple preparation stepsLow costMicrobiological testing/measurementTrichinella speciesTetrabromophenol Blue
The invention provides a kit for detecting cysticercus cellulosae, swine trichinella and swine toxoplasmosis. The kit comprises sterile deionized water, polymerase chain reaction (PCR) liquid, thermus aquaticus deoxyribonucleic acid (Taq DNA) polymerase, a GoldView DNA dye, bromophenol blue sample loading buffer solution, a standard substance and a reference substance. In the detection kit provided by the invention, DNA of the cysticercus cellulosae, the swine trichinella and the swine toxoplasmosis in a specimen to be detected can be detected quickly and accurately only by one reaction, and the kit can also be used for the molecular epidemiological survey and curative effect monitoring of cysticercus cellulosae, swine trichinella and swine toxoplasmosis infection. The method has simple preparation steps and is low in cost, easy to operate, time-saving and labor-saving, a large number of reagents and consumables are saved, and the work efficiency is improved. The method is high in detection sensibility, specificity and accuracy, and the false positive rate is reduced.
Owner:ZHEJIANG UNIV
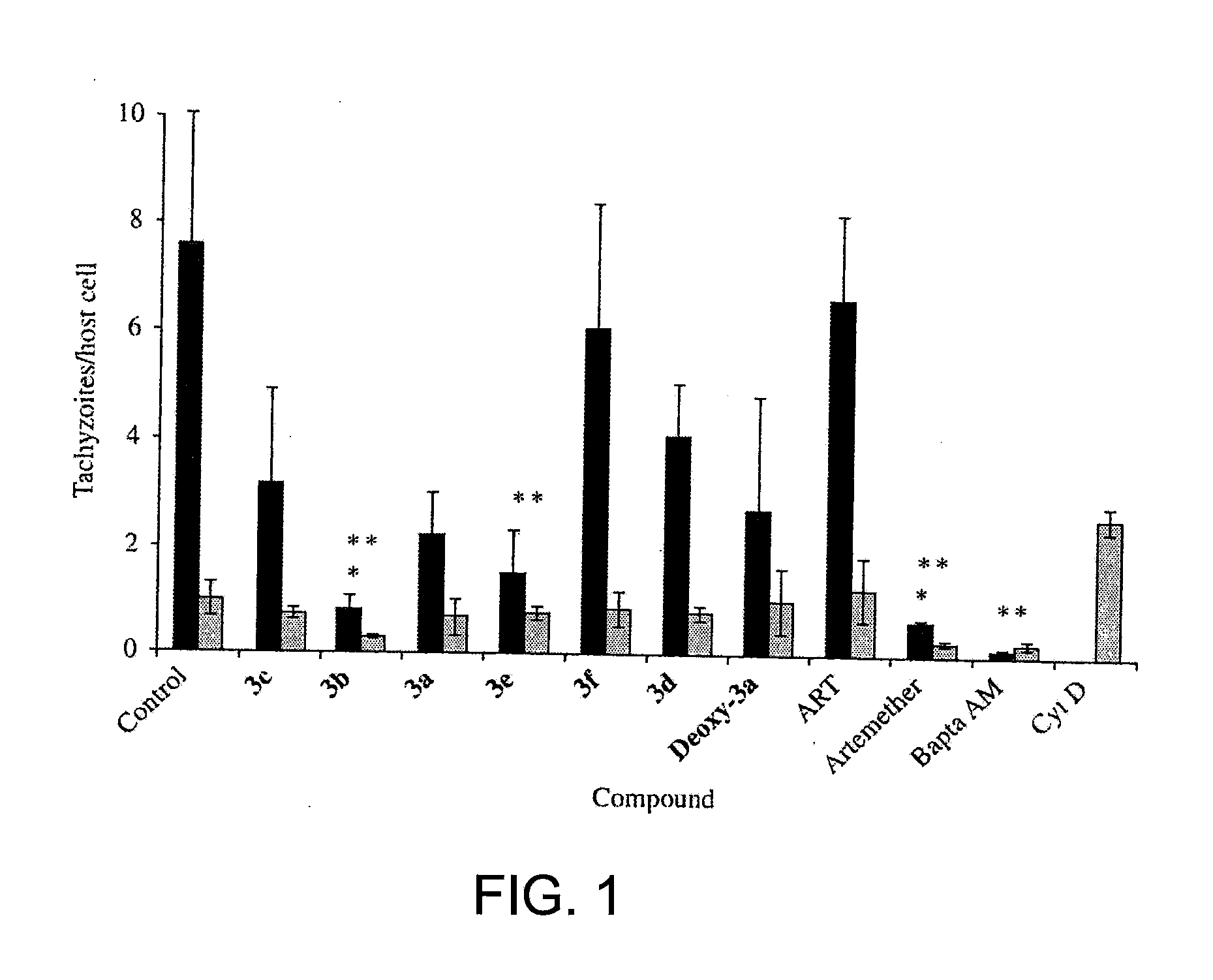
Artemisinin Derivatives
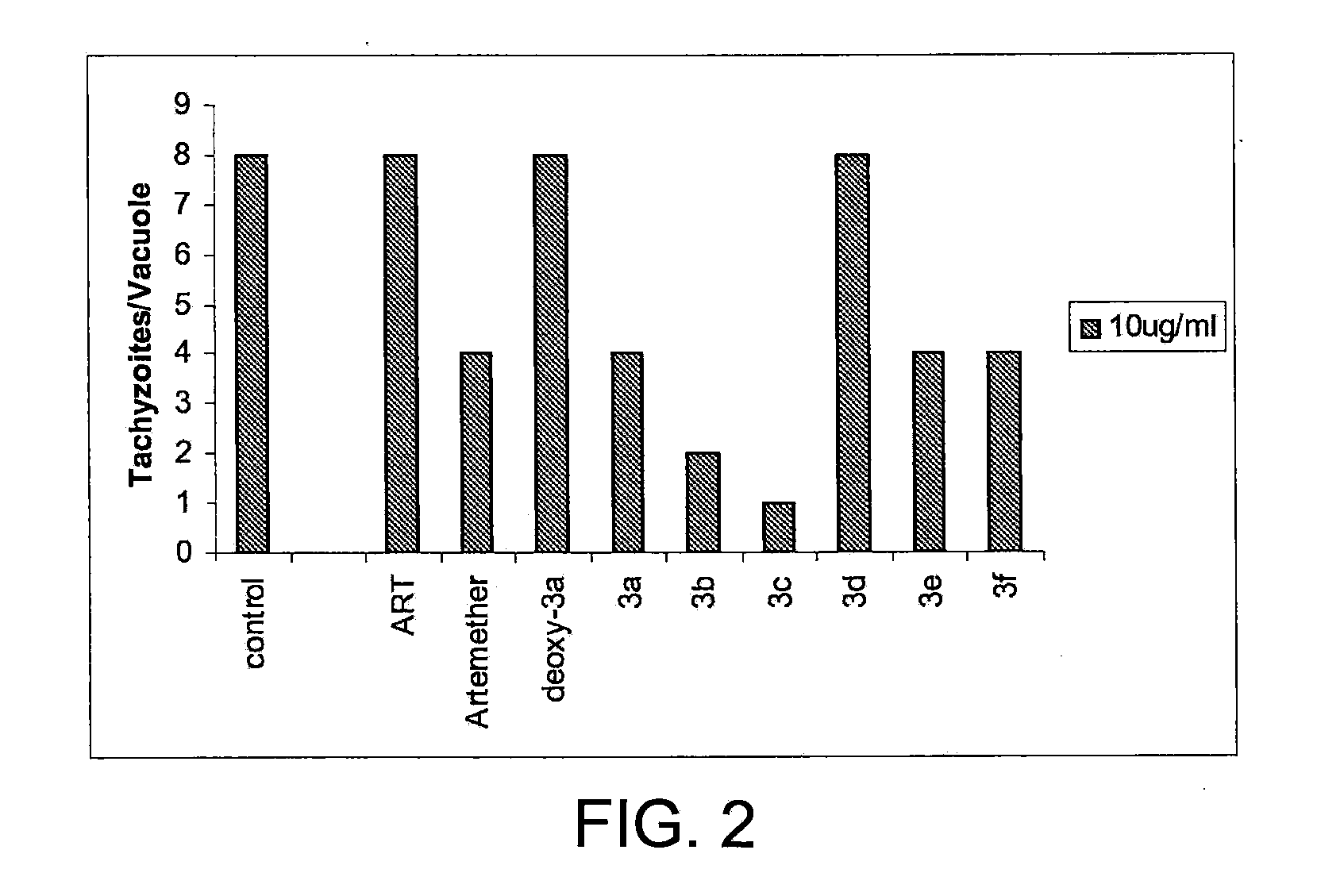
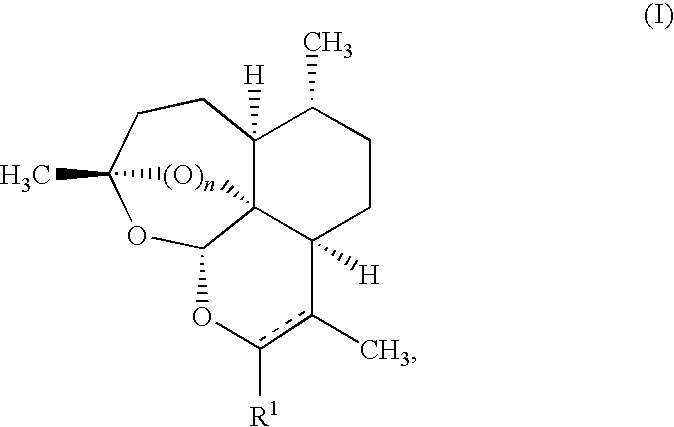
This disclosure provides improved derivatives of artemisinin; pharamaceutical compositions containing these compounds; methods for preparing these compounds and compositions; methods of using these compounds and compositions for preventing, controlling or treating infectious diseases including but not limited to parasitic infectious diseases such as T. gondii infection, trypanosome parasite infection, plasmodia parasite infection, and cryptosporidium parasite infection; methods for preventing, controlling or treating toxoplasma infection; and methods for treating psychiatric disorders associated with toxoplasma infection including but not limited to schizophrenia using the disclosed compounds and compositions alone or in combination with one or more antipsychotic drugs.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
A diagnosis antigen for Toxoplasma gondii infection and a preparation method and applications thereof
ActiveCN103235119ASolve undetectable shortcomingsLower requirementMicrobiological testing/measurementFermentationAntigenSerodiagnoses
The present invention discloses a diagnosis antigen for Toxoplasma gondii infection and a preparation method and applications thereof, belonging to the technical field of biological veterinary drugs. An antigen GRA8 suitable for diagnosis for Toxoplasma gondii infection is screened out by using methods combining Western blot, two-dimensional electrophoresis, and MALDI-TOF-TOF tandem mass spectrometry. The antigen can be used for a variety of molecular biology diagnosis and serological diagnosis for the Toxoplasma gondii infection.
Owner:NANJING AGRICULTURAL UNIVERSITY
Vaccine for preventing toxoplasma infection and application thereof
ActiveCN103330947AInfection Prevention and ControlImprove immunityProtozoa antigen ingredientsGenetic material ingredientsAntigenImmune effects
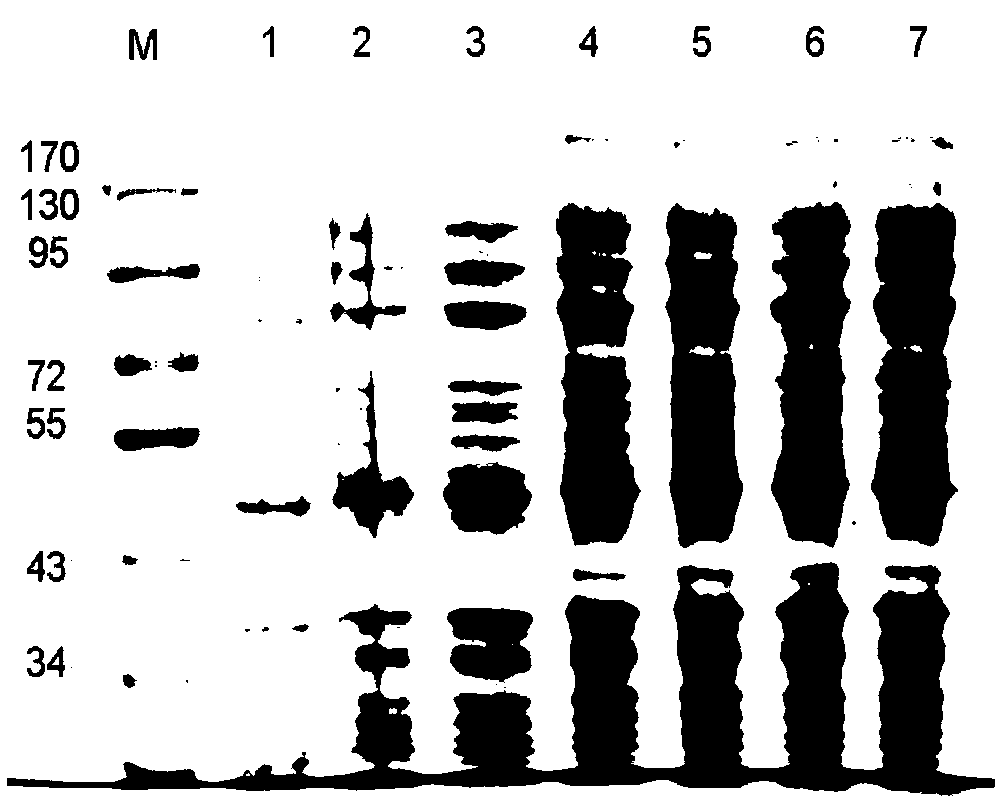
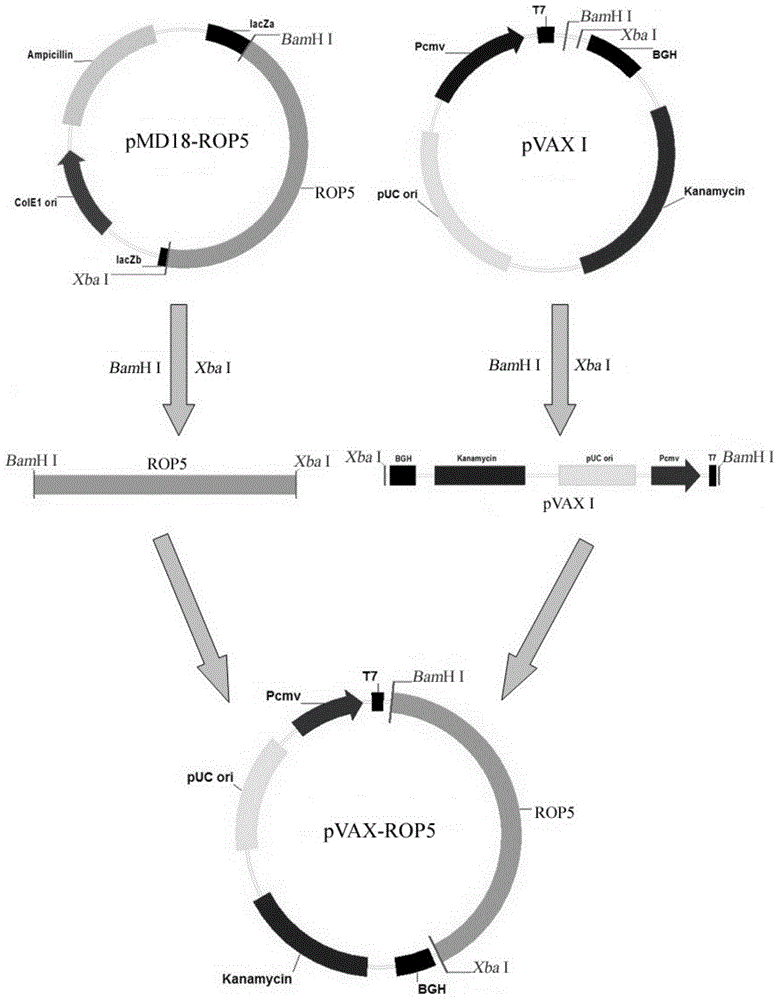
The invention provides a vaccine for preventing toxoplasma infection. The vaccine contains nucleotide sequences selected from the followings: 1) 736-2385th nucleotide sequences as shown in SEQ ID No.1, and / or 759-2411th nucleotide sequences as shown in SEQ ID No.3; 2) nucleotide sequences complementary with the nucleotide sequences as shown in 1); and 3) nucleotide sequences which perform substituting, deletion and addition modification on one or a plurality of basic groups of the nucleotide sequences as shown in 1) or 2). The vaccine has the capability of effectively preventing and controlling the infection of a toxoplasma animal model (Kunming mice), and the combination of recombinant plasmids can effectively improve the immune effect of a single-gene vaccine, therefore, plasmids pVAX-ROP5 and pVAX-GRA15 can be used as DNA (Deoxyribose Nucleic Acid) vaccine candidate antigens for preventing the toxoplasma infection; and a combined immune vaccine of a recombinant plasmid based on such two genes can be used as a cocktail composite vaccine for preventing the toxoplasma infection.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Vaccine for prevention of toxoplasma infection as well as preparation method and application of vaccine
InactiveCN103937818AInfection Prevention and ControlProlong survival timeProtozoa antigen ingredientsGenetic material ingredientsAntigenNucleotide
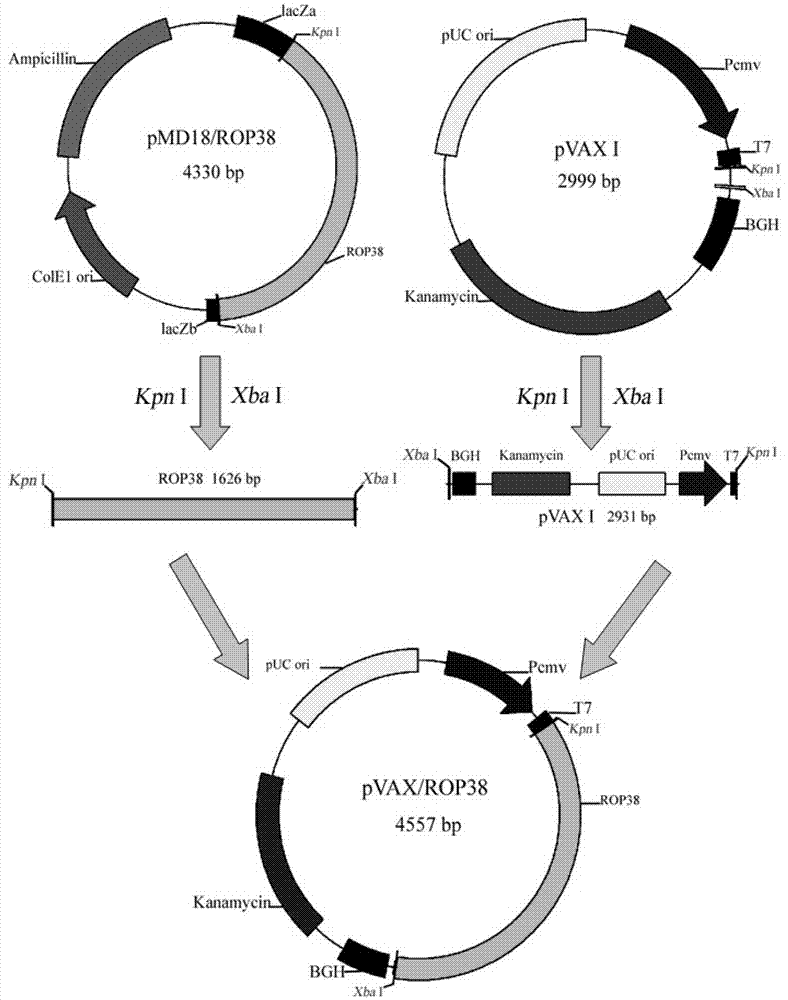
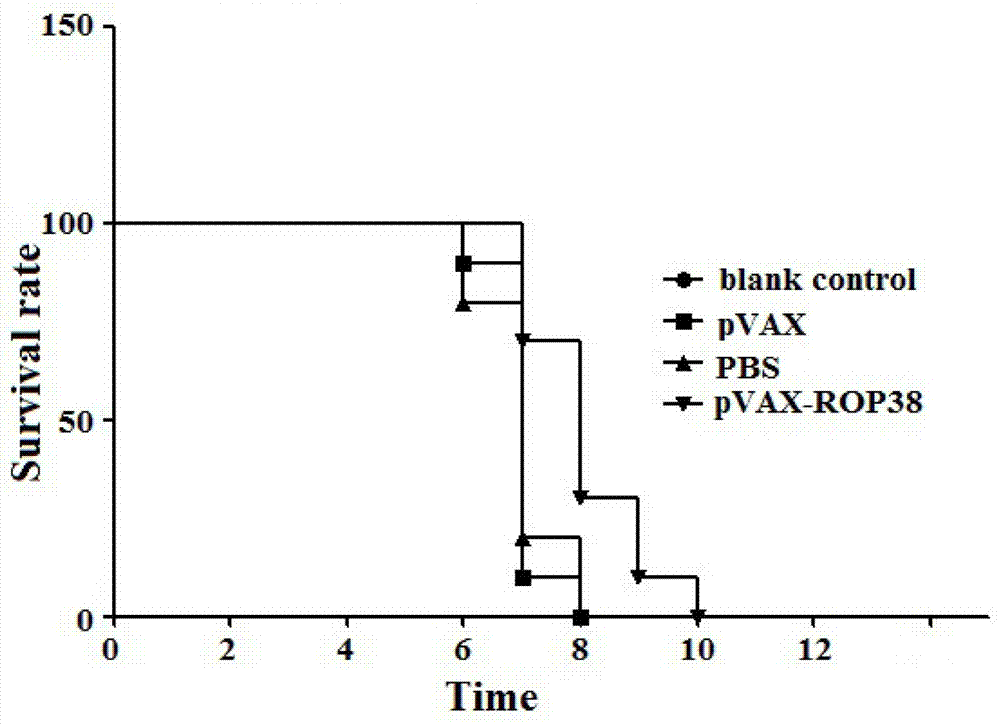
The invention provides a nucleotide sequence for prevention of toxoplasma infection. The nucleotide sequence for prevention of toxoplasma infection contains nucleotide sequences selected from (1) a nucleotide sequence as shown in SEQ ID No.1 of a sequence table, (2) a nucleotide sequence complementary with the nucleotide sequence as shown in the (1), and (3) a nucleotide sequence obtained by replacing, deleting, adding and modifying one or multiple of basic groups of the nucleotide sequence as shown in the (1) or (2) and having a function of preventing toxoplasma infection. The invention also provides a vaccine which comprises the nucleotide sequence and a medically acceptable carrier. The plasmid DNA vaccine pVAX-ROP38 can be used for effectively preventing and controlling infection of toxoplasma animal models (Kunming strain mouse), namely can be used for effectively prolonging the survival time of the mouse and reducing the worm burden of brain capsules. Therefore, the plasmid pVAX-ROP38 can serve as a DNA vaccine candidate antigen for resisting toxoplasma infection.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Dog toxoplasma gondii antibody indirect ELISA detection kit
InactiveCN104965086AImproving immunogenicityImprove protectionBiological material analysisBiological testingGondii toxoplasmaEpidemiologic survey
The invention discloses a dog toxoplasma gondii antibody indirect ELISA detection kit, and belongs to the technical field of biological inspection and quarantine. According to the dog toxoplasma gondii antibody indirect ELISA detection kit, toxoplasma gondii ROP5 recombinant protein is taken as a detection antigen. According to the dog toxoplasma gondii antibody indirect ELISA detection kit, toxoplasma gondii recombinant protein ROP5 is obtained via gene cloning and prokaryotic expression; and a detection method comprises following steps: establishment of an indirect ELISA detection method, specificity testing, sensitivity testing, and comparison with other dog toxoplasma gondii detection kits. Large-scale standardized production of an envelope antigen of the dog toxoplasma gondii antibody indirect ELISA detection kit can be realized; detection specificity is high; sensitivity is high; repeatability is high; result determination is objective; operation process is simple; and large-scale detection can be realized. The dog toxoplasma gondii antibody indirect ELISA detection kit is suitable for dog toxoplasmosis epidemiological investigation, and detection and clinical diagnosis of dog toxoplasma gondii infection; and a rapid, simple, and specific detection method is provided for diagnosis and prevention, and epidemiological investigation of dog toxoplasmosis.
Owner:SOUTH CHINA AGRI UNIV
Immunologic adjuvant for preventing toxoplasma infection and application of immunologic adjuvant
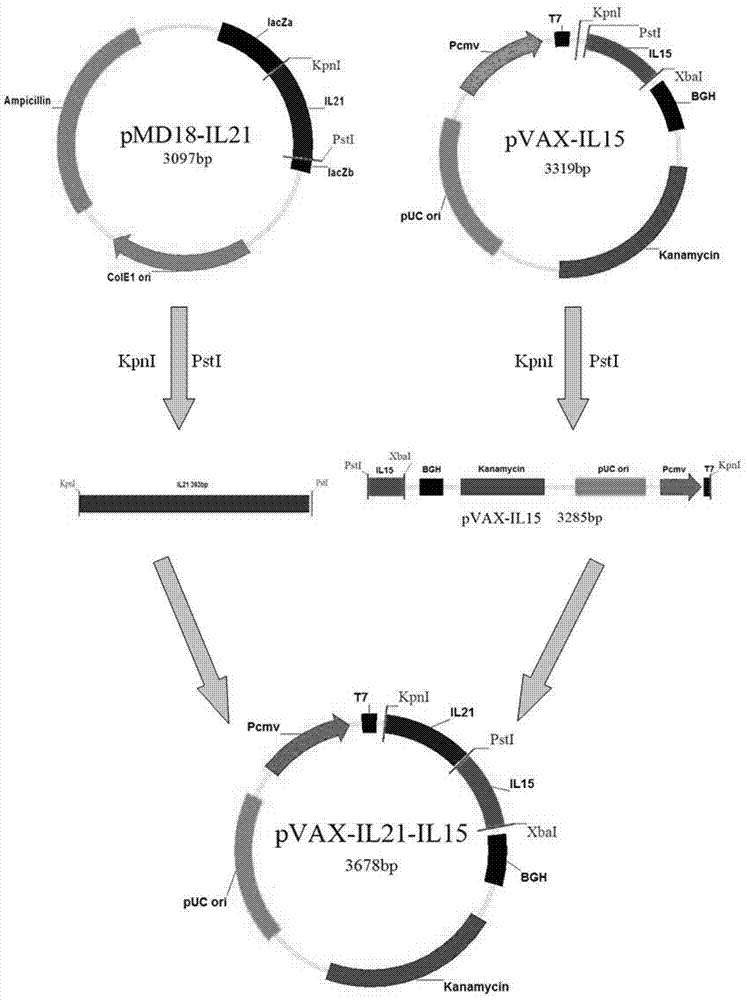
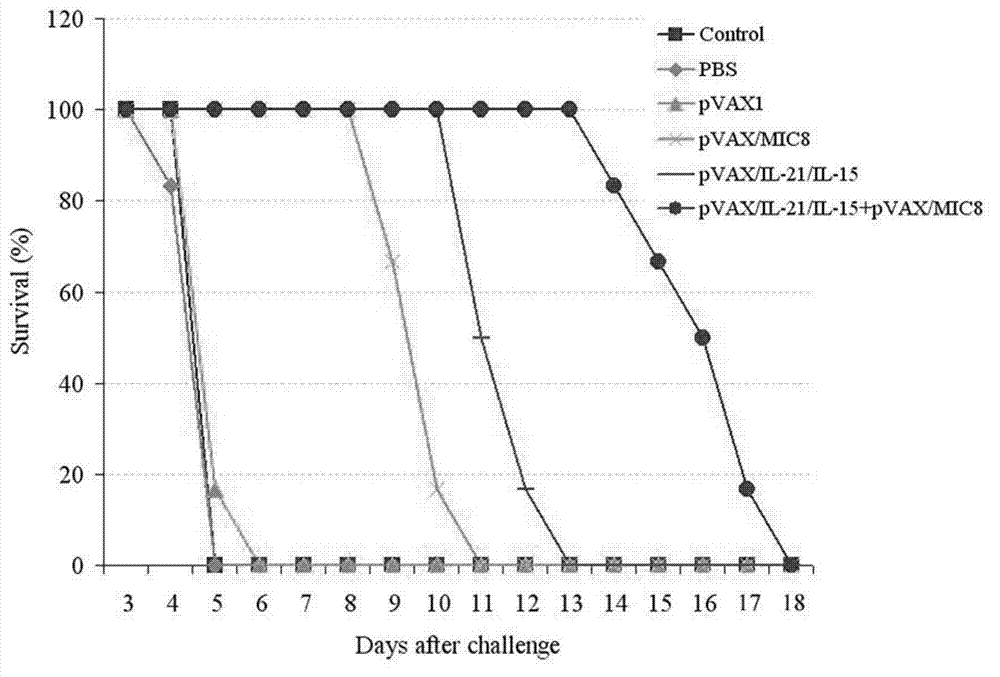
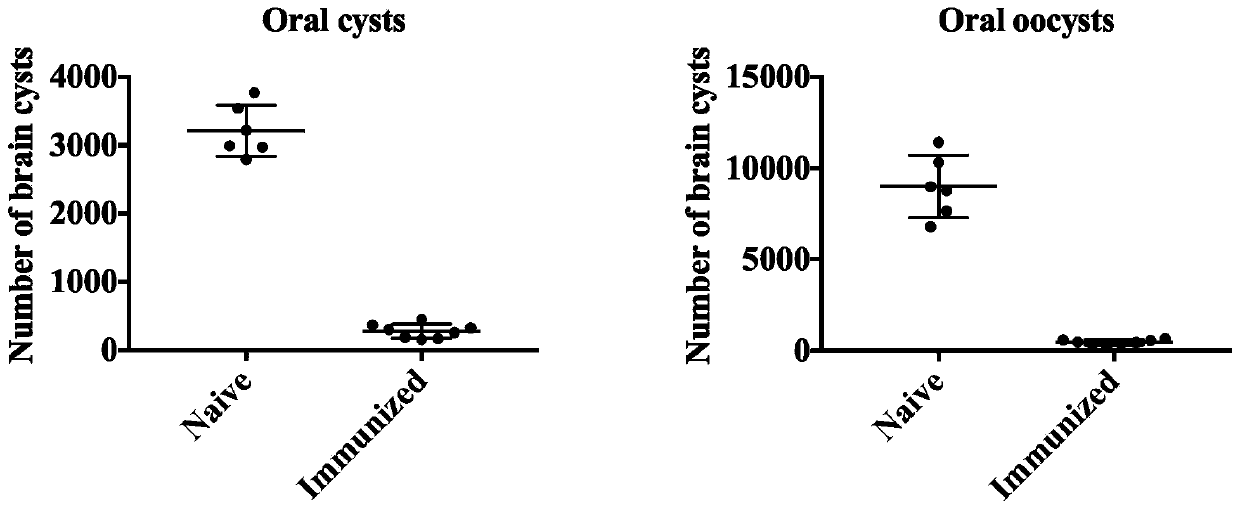
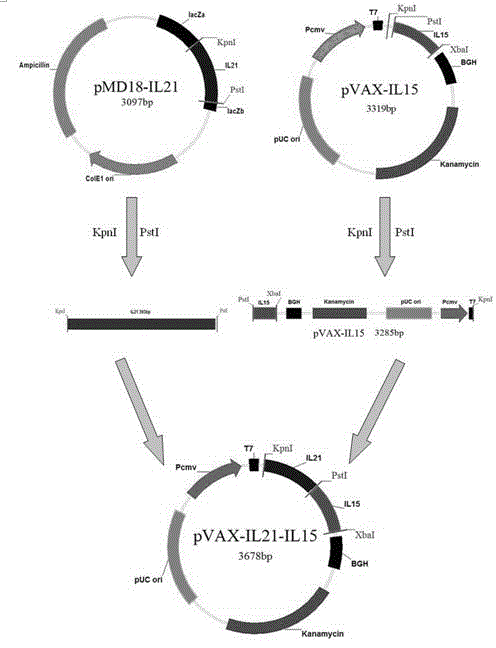
The invention discloses an immunologic adjuvant for preventing toxoplasma infection. The immunologic adjuvant is characterized in that the immunologic adjuvant is recombinant plasmids pVAX-IL21-IL15 formed by fusing interleukins IL21 and IL15 by using pVAX1 plasmids as carriers, and the base sequence of the immunologic adjuvant is represented by SEQ ID NO:1. The immunologic adjuvant for preventing the toxoplasma infection has a function of obviously improving the immune effect of MIC8 genes, so that the immune effect of an immune prevention vaccine against toxoplasma can be obviously enhanced.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Vaccine for preventing toxoplasma infection as well as preparation method and application thereof
InactiveCN104152466AInfection Prevention and ControlProlong survival timeProtozoa antigen ingredientsGenetic material ingredientsAntigenNucleotide
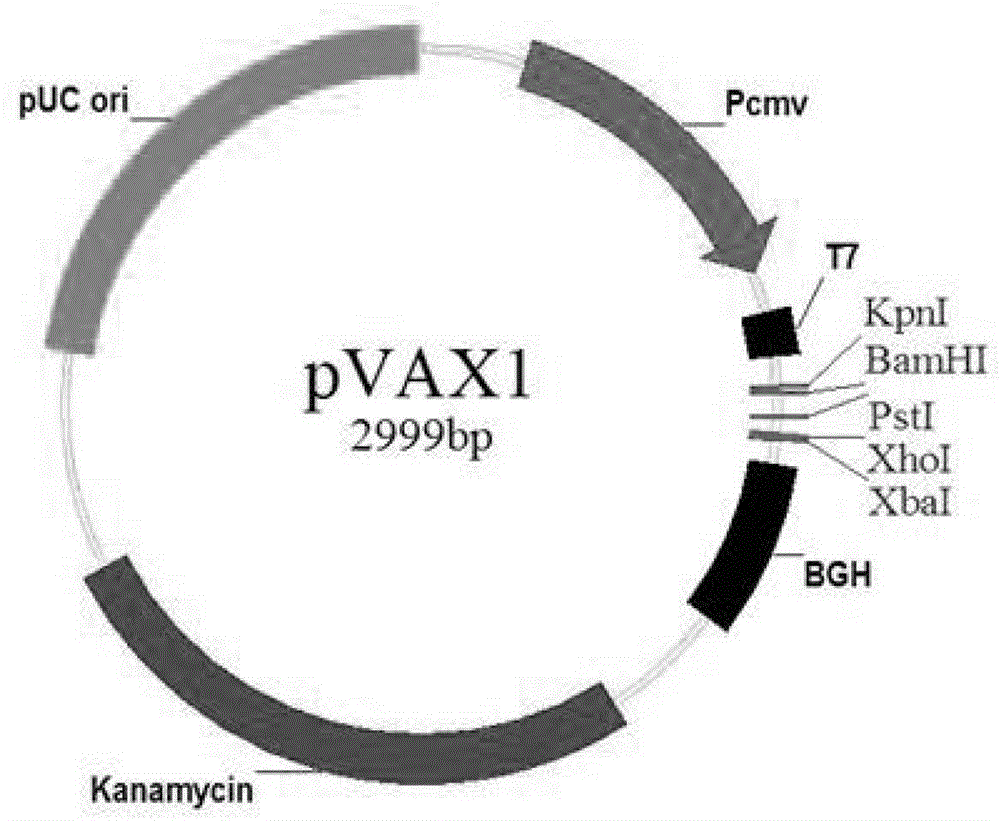
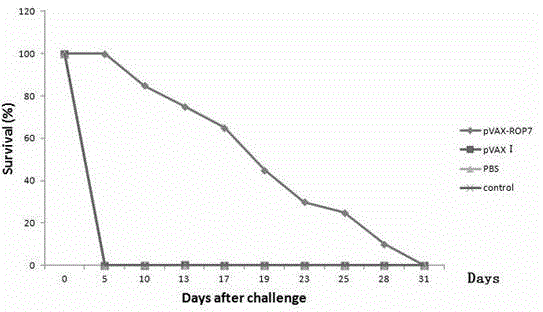
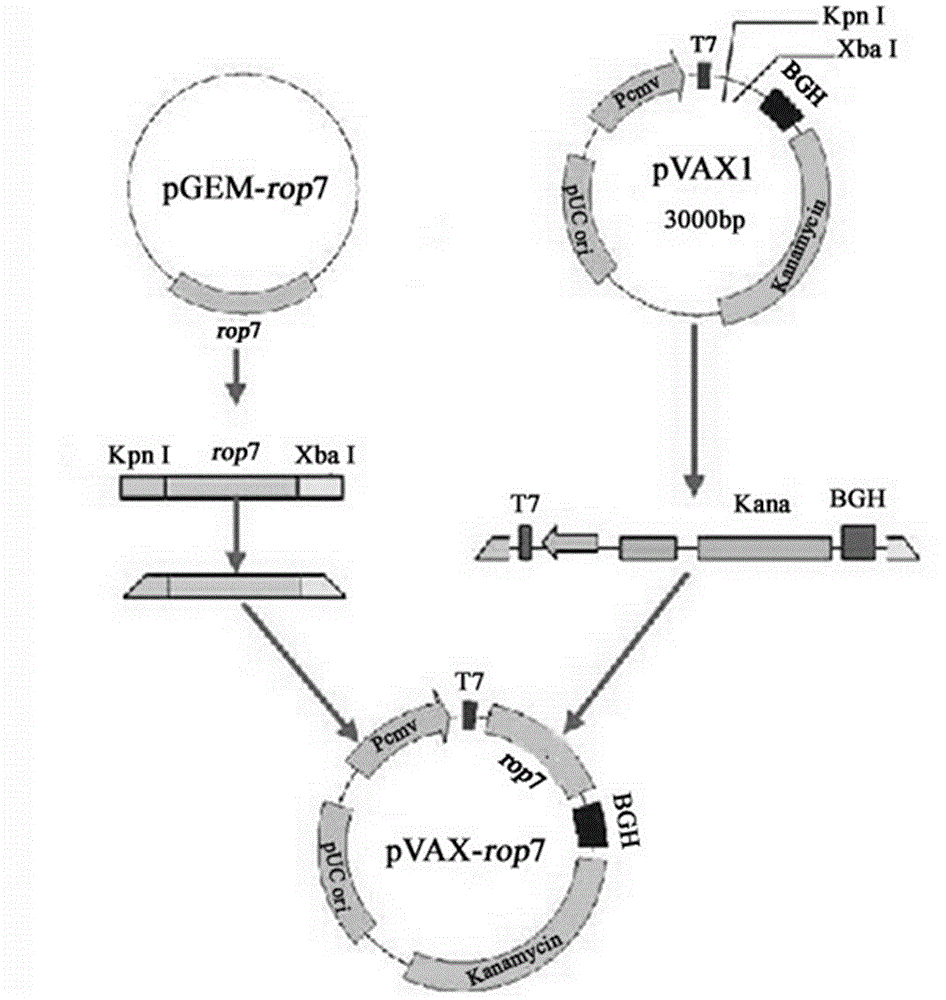
The invention firstly provides a nucleotide sequence for preventing toxoplasma infection. The nucleotide sequence is selected from one of the following nucleotide sequences: (1) a nucleotide sequence as shown in SEQ ID No.1 in a sequence table, (2) a nucleotide sequence which is complementary with the nucleotide sequence in (1) and (3) a nucleotide sequence which is obtained by substituting, deleting and modifying one or more basic groups of the nucleotide sequence as shown in (1) or (2) and has a function of preventing toxoplasma infection. The invention also provides a vaccine comprising the nucleotide sequence and a medically acceptable carrier. The plasmid DNA vaccine pVAX-ROP7 provided by the invention can be used for effectively preventing and controlling toxoplasma animal model (Kunming mouse) infection, namely effectively prolonging the survival time of a rat and reducing the polypide quantity of a brain cyst. Therefore, the plasmid pVAX-ROP7 can be used as a candidate antigen of DNA vaccine to prevent toxoplasma infection.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Nucleotide sequence for preventing sheep toxoplasmosis infection, vector, protein, vaccine, preparation method and application thereof
InactiveCN106397563AStrong cellular immunityStrong humoral immunityProtozoa antigen ingredientsGenetic material ingredientsInflammatory factorsEnzyme digestion
The invention provides a nucleotide sequence for preventing sheep toxoplasmosis infection, a vector, a protein, a vaccine, a preparation method and application thereof. The nucleotide sequence comprises the following nucleotide sequences: (1) nucleotide sequence shown in SEQ ID NO.1; (2) nucleotide sequence which is complementary to the nucleotide sequence shown in (1); and (3) nucleotide sequence obtained by subjecting the nucleotide sequence in the (1) or (2) to replacing, deleting and adding and modifying of one or several base groups. The invention also provides the vaccine which is prepared by that toxoplasma antigen is subjected to double-enzyme digestion and then is connected with a pET28a expression vector, and then is transformed into a BL21 (DE3) engineering bacteria, high-efficiency expression is induced, the expression product is purified to obtain soluble proteins, and finally the soluble proteins are added with 206 adjuvant to obtain the vaccine. The prepared subunit inactivated vaccine has relatively strong cell immunity and humoral immunity effect, the congenital immune response of the body can be induced, various cell inflammatory factors are secreted, and therefore the toxoplasmosis is effectively prevented.
Owner:AIKAN BIOTECH TIANJIN CO LTD
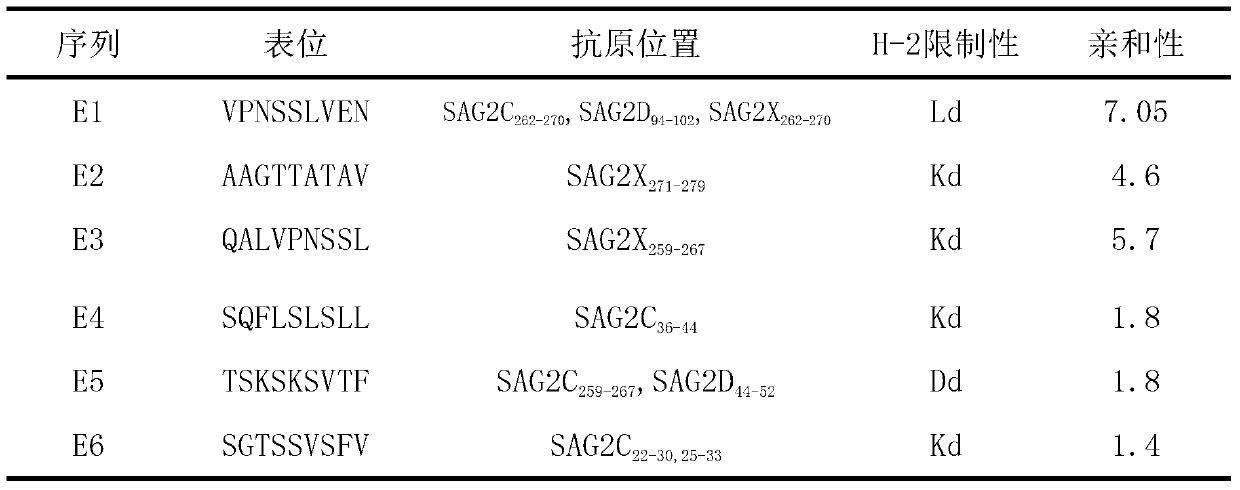
CD8+T cell dominant epitopes based on toxoplasmagondii bradyzoite antigens
ActiveCN103275182AProtozoa antigen ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsBALB/cActive immunization
The invention discloses two CD8+T cell dominant epitopes of VPNSSLVEN and SQFLSLSLL based on toxoplasmagondii bradyzoite specific surface antigens, wherein six CD8+T cell epitopes with H-2 restriction and high affinity are screened according to the protein sequences of the bradyzoite specific surface antigens of SAG2C, -2C and -2X; BALB / c mice are actively immunized by the screened epitope polypeptides; determinations for a lymphopoiesis level and a cell factor content, and an immune protection evaluation for epitope vaccines are performed after the immunization. The result indicates that powerful cell immunization can be generated by inducing the mice via the epitope vaccinesm, wherein the two antigen peptides of VPNSSLVEN and SQFLSLSLL are provided to be great in the capacity of stimulating T cell proliferation, as well as capable of inducing the mice to secrete protective cell factors and immune protection, being used as the epitope vaccines effectively resisting toxoplasmagondii infection, and reducing an encystations rate in the brain tissue of a host.
Owner:SHANDONG UNIV
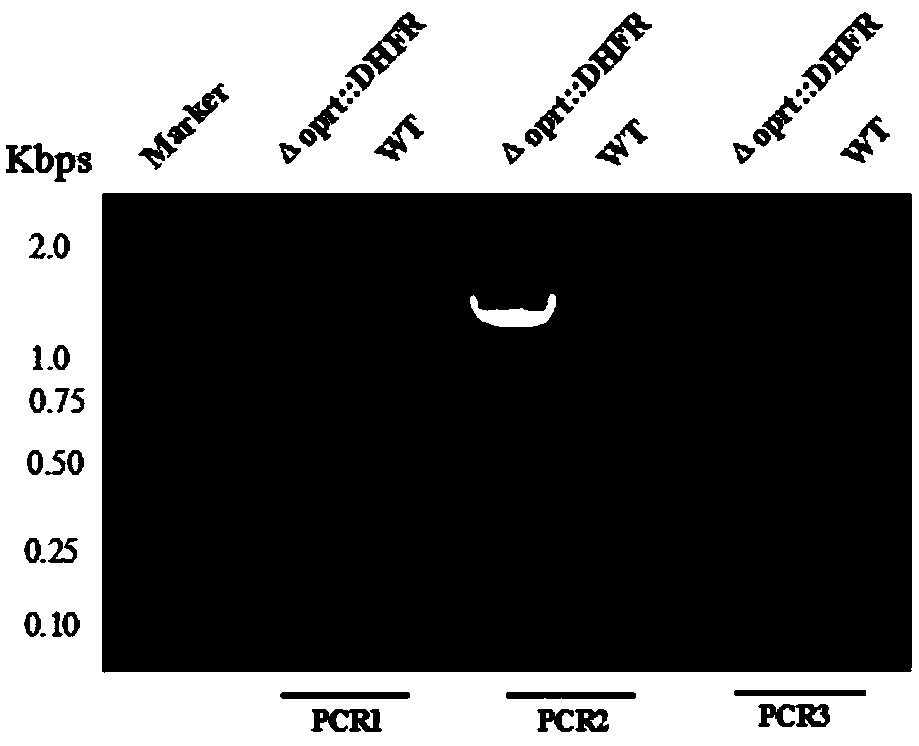
Toxoplasma gondii attenuated live vaccine with deficiency of OMPDC and LDH1 genes
ActiveCN108434447APrevention of acute and chronicPrevent congenital infectionProtozoa antigen ingredientsStable introduction of DNALactate dehydrogenaseGondii toxoplasma
The invention discloses an attenuated live vaccine capable of preventing toxoplasma gondii infection. The vaccine contains a toxoplasma gondii vaccine strain with simultaneous deficiency of the orotidine-5-phosphate decarboxylase gene and the lactic dehydrogenase 1 gene, wherein the nucleotide sequence of the orotidine-5-phosphate decarboxylase gene is as shown in SEQ ID NO:1, and the nucleotide sequence of the lactic dehydrogenase 1 gene is as shown in SEQ ID NO:2. The attenuated live vaccine provided by the invention has the advantages that basically no virulence exists, the immunocompetencefor toxoplasma gondii of animals can be improved, the acute and chronic as well as congenital infection of toxoplasma gondii can be prevented, the vaccine strain cuts off the drug resistance screening label, and thus the vaccine strain has the potential of being prepared into the genetically engineered vaccine for resisting toxoplasma gondii.
Owner:HUAZHONG AGRI UNIV
Specific primers, probes, kit and chip for gene detection of Toxoplasma gondii
PendingCN110982880AConvenient diagnosis and treatmentEasy to monitorMicrobiological testing/measurementDNA/RNA fragmentationGondii toxoplasmaVirology
The invention relates to the field of molecular biology, and provides specific primers for detecting Toxoplasma gondii. The sequence of an upstream primer in the specific primers is as shown in SEQ IDNo. 1; the sequence of a downstream primer in the specific primers is as shown in SEQ ID No. 2. The invention also provides probes for detecting Toxoplasma gondii. The sequences of the probes are asshown in SEQ ID No. 3-6. The invention further provides a kit for detecting Toxoplasma gondii. The kit comprises the primers and the probes for detecting Toxoplasma gondii. The invention further provides a chip for detecting Toxoplasma gondii. The chip comprises the probes for detecting Toxoplasma gondii. According to the invention, gene chip detection is carried out on Toxoplasma gondii by usingthe primers and the probes, and differential diagnosis can be specifically carried out on Toxoplasma gondii infection conditions.
Owner:中国疾病预防控制中心寄生虫病预防控制所国家热带病研究中心
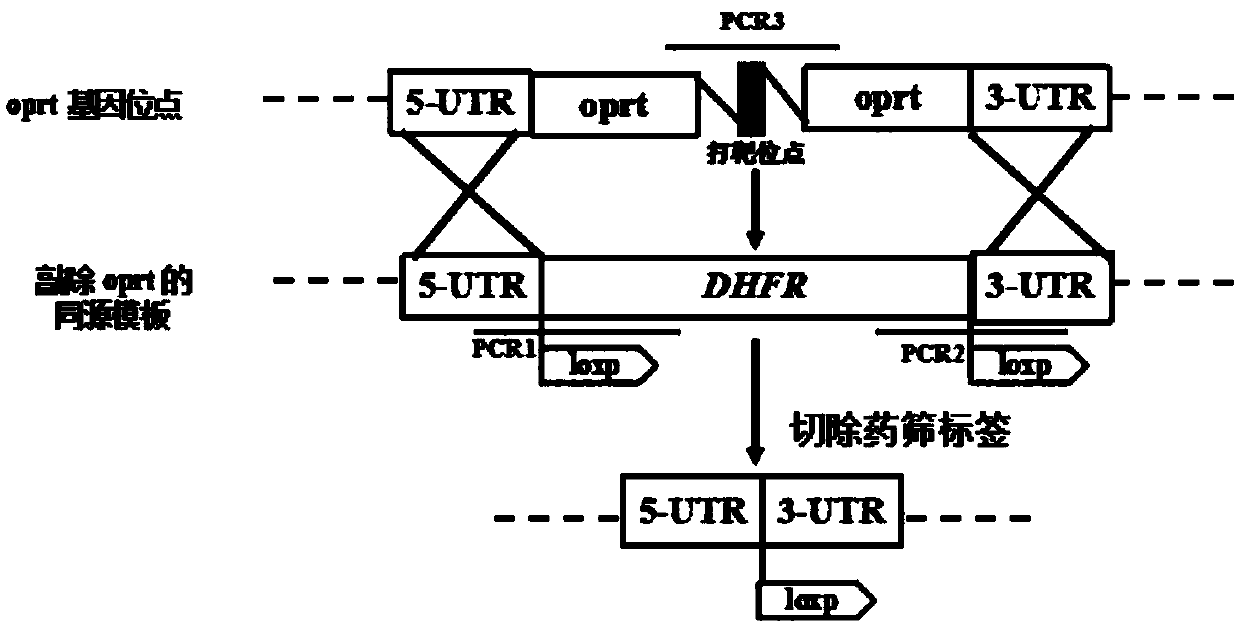
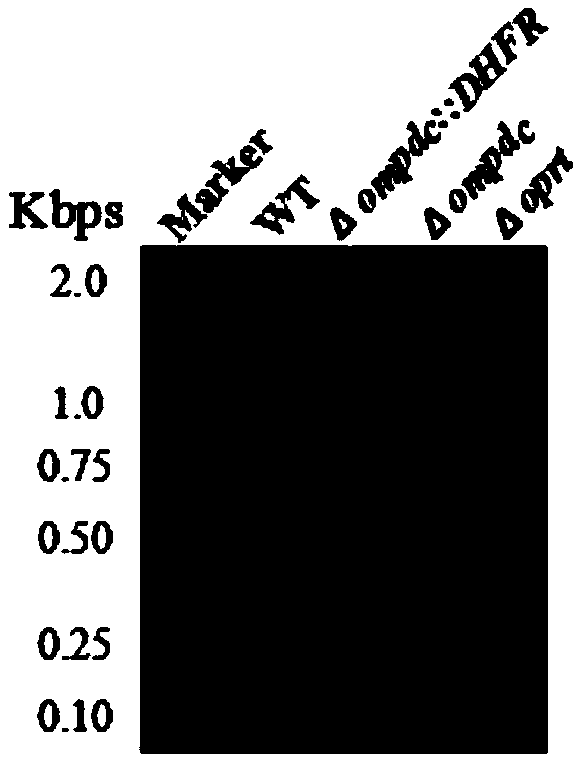
Toxoplasma gondii attenuated live vaccine with deficiency of OPRT and LDH1 genes and preparation method of toxoplasma gondii attenuated live vaccine
ActiveCN108379570AAvoid infectionImprove immunityProtozoa antigen ingredientsProtozoaLactate dehydrogenaseVirulent characteristics
The invention discloses an attenuated live vaccine capable of preventing toxoplasma gondii infection. The vaccine contains a toxoplasma gondii vaccine strain with deficiency of both the orotate phosphoribosyltransferase (OPRT) gene and the lactate dehydrogenase 1 (LDH1) gene, wherein the nucleotide sequence of the OPRT gene is as shown in SEQ ID NO:1, and the nucleotide sequence of the LDH1 gene is as shown in SEQ ID NO:2. According to the attenuated toxoplasma gondii live vaccine used for preventing the animal toxoplasmosis provided by the invention, for the vaccine strain, the OPRT and LDH1genes are knocked out, thus the vaccine strain has the advantages that the virulence is weak and basically no pathogenicity is caused, and the vaccine strain can be used for preventing toxoplasma gondii infection of animals, and has the potential of being prepared into a genetic engineering vaccine for resisting toxoplasma gondii.
Owner:HUAZHONG AGRI UNIV
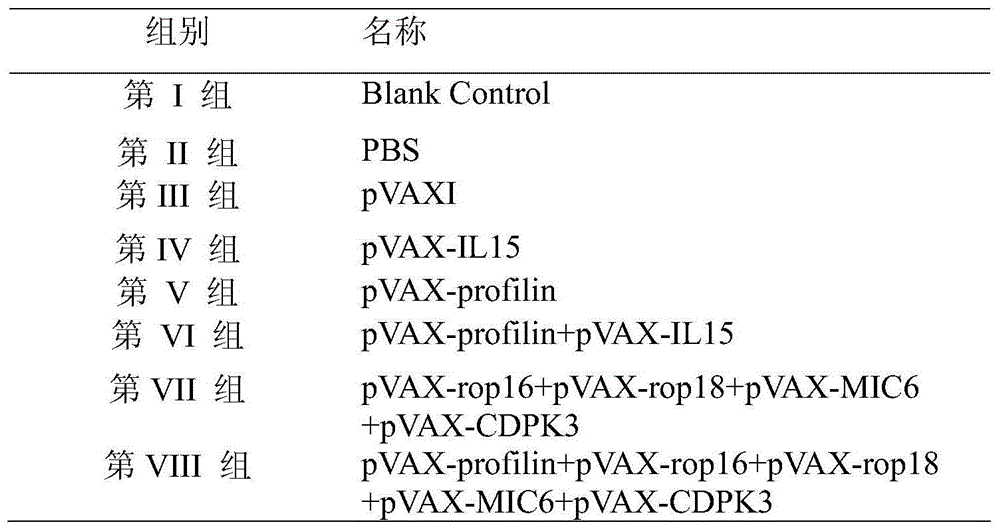
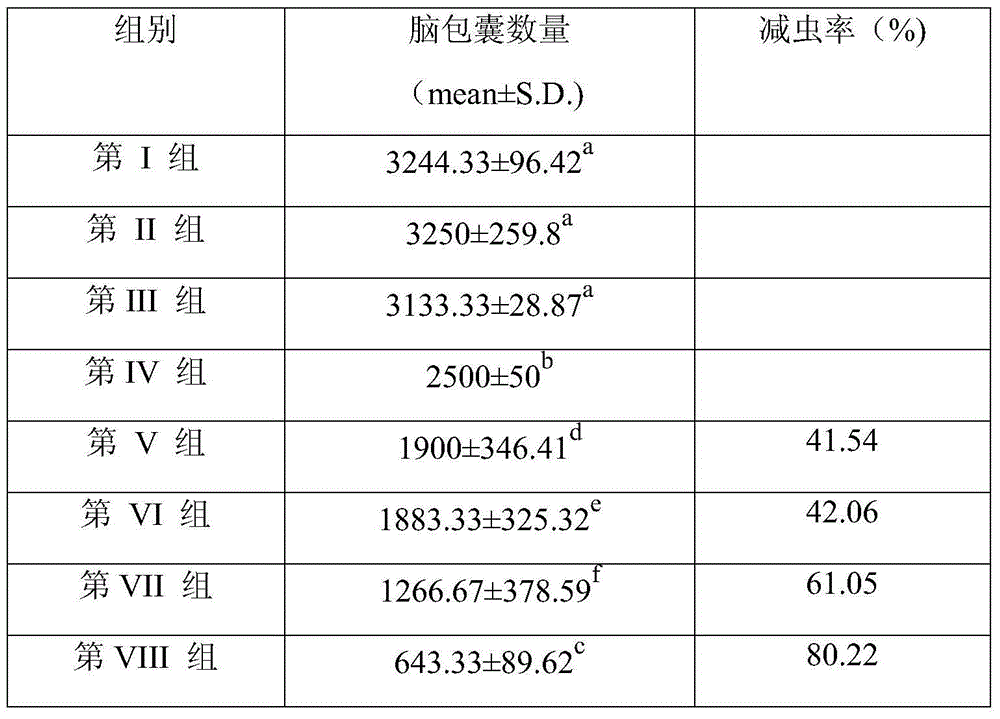
Nucleotide sequence for preventing infection of toxoplasmas and application of nucleotide sequence
InactiveCN104830850APromote aggregationInhibitionOrganic active ingredientsPeptide/protein ingredientsImmune effectsNucleotide
The invention provides a nucleotide sequence for preventing infection of toxoplasmas. The nucleotide sequence contains any one group of the following nucleotide sequences: (1) a nucleotide sequence of profilin; (2) nucleotide sequences of profilin, rop16, rop18, MIC6 and CDPK3; (3) nucleotide sequences of profilin and IL15; (4) nucleotide sequences of rop16, rop18, MIC6 and CDPK3; (5) complementary sequences of the nucleotide sequences from (1) to (4); and (6) nucleotide sequences prepared through carrying out substitution, deletion, addition and modification on nucleotide sequences from (1) to (5). By virtue of a pVAX-profilin vaccine and a combined vaccine, the generation of brain cysts of the toxoplasmas can be effectively inhibited, and a relatively good immune effect is achieved.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Attenuated live vaccine used for preventing toxoplasma infection and application of attenuated live vaccine
InactiveCN107308444AAvoid infectionPrevent acute infectionProtozoa antigen ingredientsAntiparasitic agentsAcute infectionSolvent
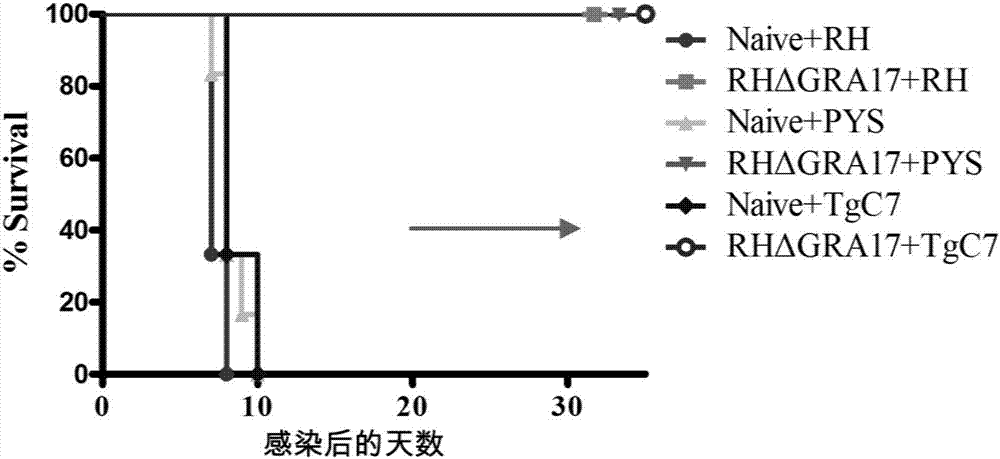
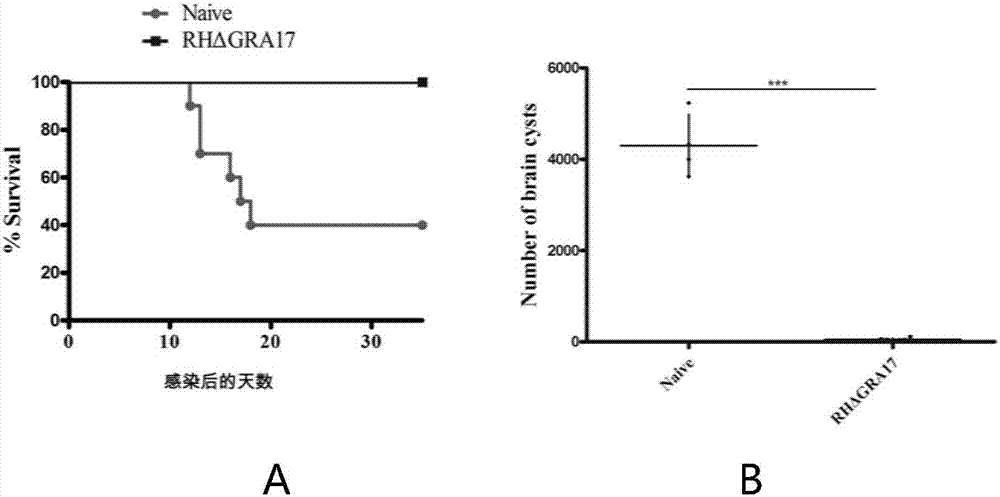
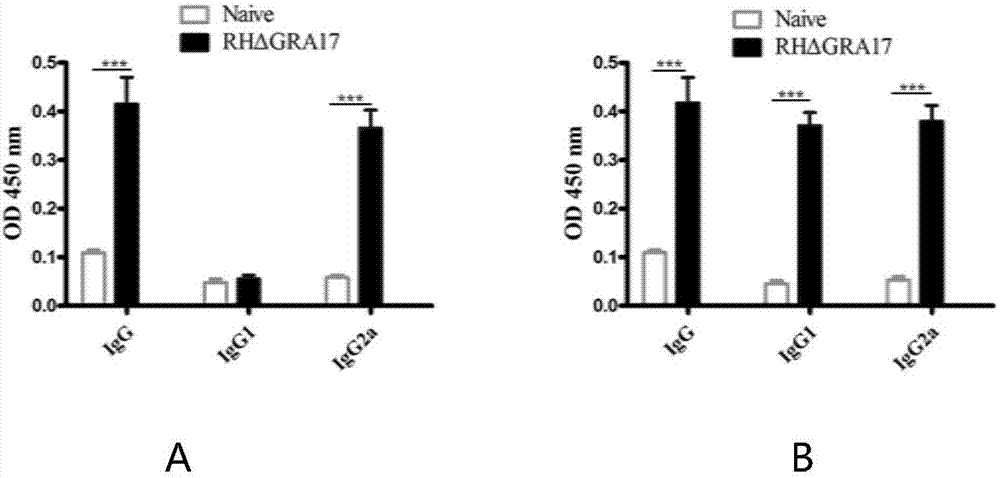
The invention discloses an attenuated live vaccine used for preventing toxoplasma infection and application of the attenuated live vaccine. The attenuated live vaccine takes PBS as a solvent to prepare a tachyzoite suspension of a toxoplasmosis attenuative strain RHdetltaGRA17. An immune preparation for the attenuated live vaccine is established, the immunization procedure and the inoculum size of the vaccine are explored, and the animal experiment shows that the attenuated live vaccine has a stronger immunoprotection function for the acute infection, the chronic infection and the congenital infection of toxoplasmosis, the normal infection of the toxoplasmosis is prevented, meanwhile, the mother-to-fetus vertical propagation of the toxoplasmosis is effectively stopped, and the vaccine has a huge application value, and can be used for preventing the acute infection, the chronic infection and the congenital infection of toxoplasmosis.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Mimotopic polypeptides of toxoplasma gondii and applications
The invention relates to a polypeptide capable of reacting specifically with an anti-Toxoplasma gondii P30 protein antibody, and comprising a peptide sequence, in which any succession of 6 contiguous amino acids exhibits at most 67 % homology with the peptide sequence of said P30 protein, identified by SEQ ID No.1, or comprising a sequence derived from said peptide sequence, and the applications of this polypeptide especially for detecting a Toxoplasma gondii infection, in a biological sample.
Owner:BIOMERIEUX SA
Semi-nested PCR method testing toxoplasma gondii
InactiveCN101245367AQuick checkAccurate detectionMicrobiological testing/measurementEpidemiologyImpurity
The invention provides a semi-nested PCR method for detecting toxoplasma gondii, which determines whether a sample is toxoplasma gondii infection positive or negative by arranging a PCR detection kit, extracting the DNA of a test sample and carrying out the semi-nested PCR amplification and the analysis of the amplified products. The method of the invention can rapidly and accurately detect the toxoplasma gondii in the test sample, and can also be used for the molecular epidemiological investigation and the efficacy monitoring of toxoplasmosis. The template DNA preparation steps of the method are simple, the cost is low, however, the conventional method needs to be processed by lysozyme, protease K, sodium dodecyl sulfate, cetyltrimethyl ammonium bromide and other reagents, the time is long and the cost is high. The method is established on the molecular biology, which can exclude the interferences of bacteria and impurity particles, thus greatly improving diagnostic accuracy and reducing false positive rate.
Owner:ZHEJIANG UNIV
Attenuated live vaccine for preventing toxoplasma gondii infection and application of attenuated live vaccine
PendingCN110384797AImprove securityGood immune protectionAntiparasitic agentsInvertebrate antigen ingredientsAcute infectionAttenuated Live Vaccine
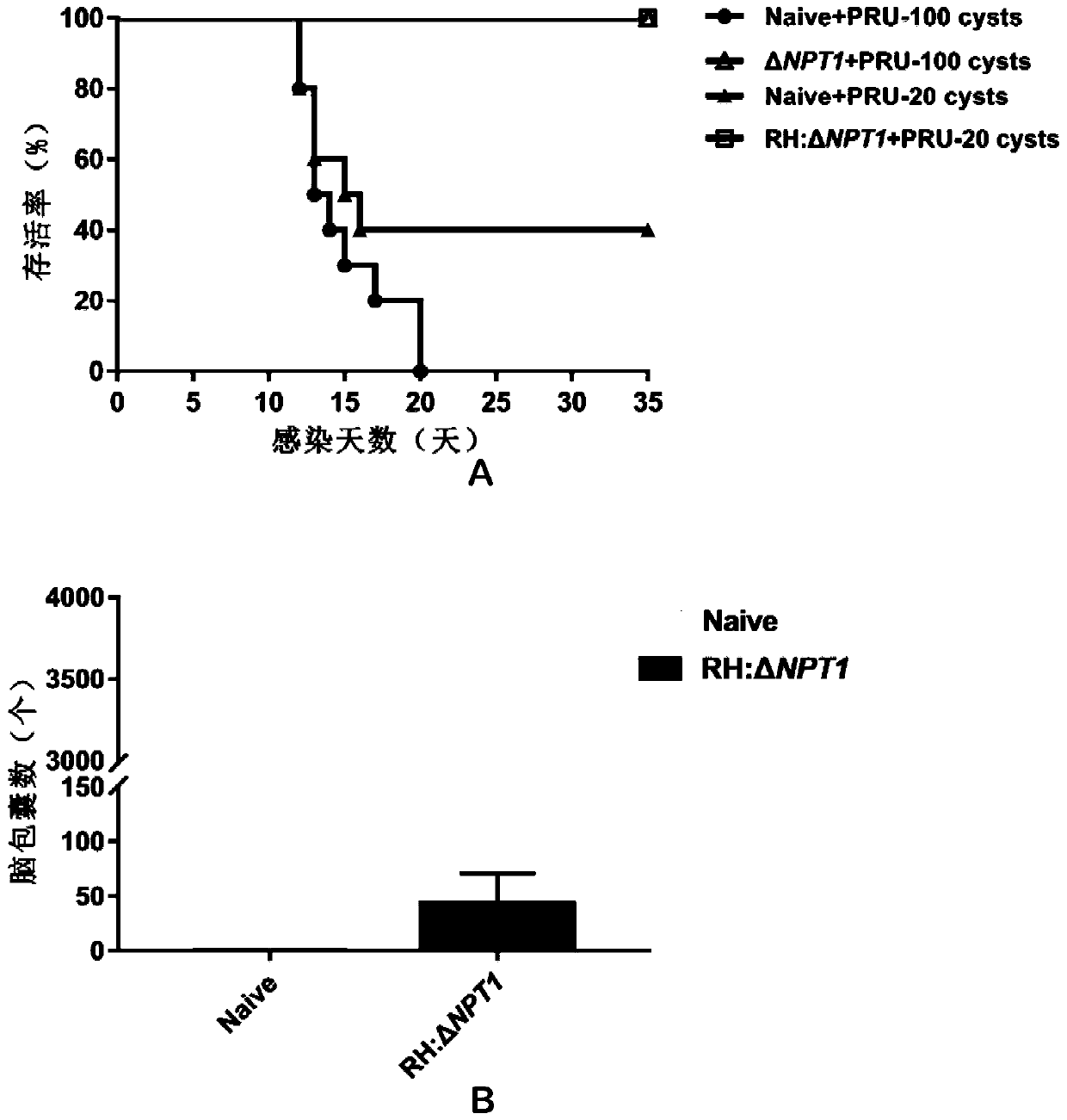
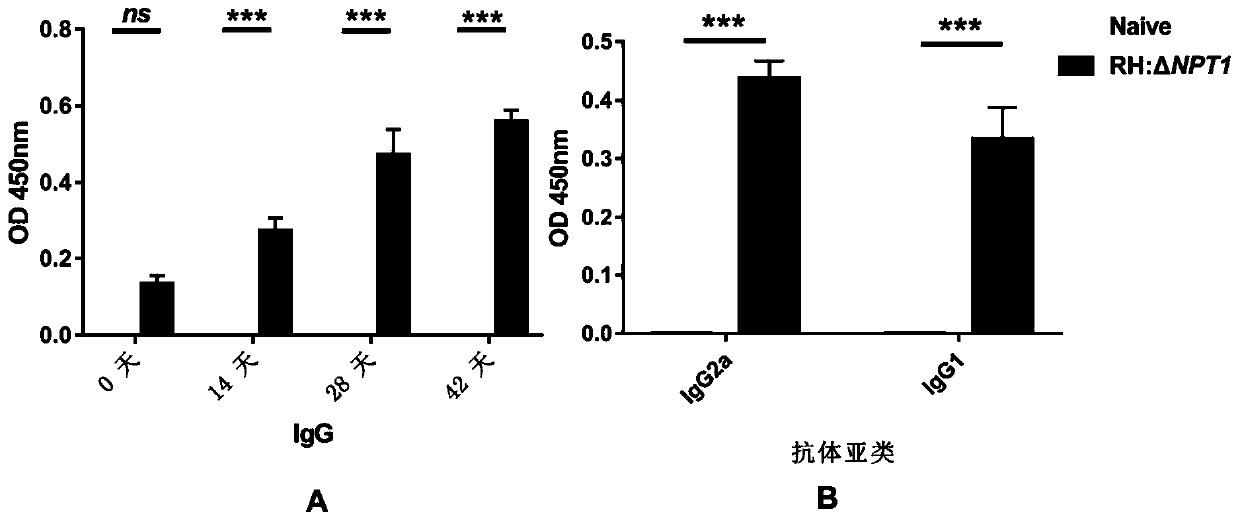
The invention discloses an attenuated live vaccine for preventing toxoplasma gondii infection and application of the attenuated live vaccine. The attenuated live vaccine is tachyzoite suspension, prepared by taking PBS as a solvent, of a toxoplasma gondii attenuated stain RH: delta gra17 delta npt 1. Toxoplasma gondii double gene knockout is conducted, the immune dosage form of the attenuated livevaccine is determined, the immune vaccination program and dosage of the attenuated live vaccine are verified, it is found that the attenuated live vaccine has a good immune protection effect on toxoplasma gondii acute infection, chronic infection and congenital infection, and toxoplasmosis can be effectively prevented.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Oral liquid for inhibiting and eliminating toxoplasma gondii and toxoplasma gondii cysts and preparation method of oral liquid
InactiveCN109908265AInhibition of reproductive activityInhibition of reproductionPharmaceutical delivery mechanismAntiparasitic agentsBiotechnologySmoked Plum
The invention discloses oral liquid for inhibiting and eliminating toxoplasma gondii and toxoplasma gondii cysts and a preparation method of the oral liquid. The oral liquid comprises, by weight, 2-4parts of Chinese wolfberries, 12-16 parts of smoked plums, 15-20 parts of the root of Indian pokeweed, 50-60 parts of herba artemisiae annuae, 10-15 parts of dandelions, 4-7 parts of rhizoma polygonati, 4-6 parts of radix bupleuri, 8-10 parts of white poria, 2-4 parts of cyrtomium fortunei, 3-5 parts of bletilla striata, 3-5 parts of branchlets and leaves of antifebrile di-chroa, 4-6 parts of fructus ulmi, 6-10 parts of frankincense, 6-8 parts of the root bark of white mulberry, 1-3 parts of white atractylodes rhizomes and 300-500 parts of purified water. The preparation method includes the following steps: extracting active ingredients of the raw materials and uniformly mixing the active ingredients with the purified water. The oral liquid can inhibit the breeding and activity of the toxoplasma gondii in human cells after being continuously taken, thereby preventing the infection of the toxoplasma gondii, treating toxoplasmosis, and expelling the cysts and pseudocysts of the toxoplasma gondii.
Owner:ZHUHAI HANTANG BIOTECH ENG CO LTD
Vaccine for preventing dog Toxoplasma gondii infection and preparation method of vaccine
ActiveCN108822200AMaintain immunogenicityPreventing Canine ToxoplasmosisProtozoa antigen ingredientsBacteriaEscherichia coliEnzyme digestion
The invention provides protein with Toxoplasma gondii immunogenicity. The protein is cyclophilin mutant protein and consists of the amino acid sequence shown in SEQ ID 2. The invention further provides nucleic acid capable of encoding the protein with Toxoplasma gondii immunogenicity and consists of the nucleic acid sequence shown in SEQ ID 1. The invention also provides a vaccine. A Toxoplasma gondii antigen gene is subjected to double enzyme digestion, then linked to prokaryotic expression vectors such as pET28a and the like and transformed into prokaryotic expression engineering bacteria such as BL21 (DE3) and the like to be induced to be highly expressed, protein obtained after purification is soluble protein, and the specific immunogenicity of the protein is maintained. The sequence of the cyclophilin protein is optimized, so that the expression amount of the cyclophilin protein in prokaryotic Escherichia coli is remarkably increased. The vaccine can be prepared by engineering strains by adding vaccine adjuvants, unique immunogenicity of the vaccine is maintained, and the vaccine is suitable for industrial production.
Owner:HAIMU ANIMAL HEALTH PROD (SHANDONG) CO LTD
Gene for preventing toxoplasma infection and application of gene
ActiveCN103333900AInfection Prevention and ControlImprove immunityHydrolasesGenetic material ingredientsAdjuvantCalcium-dependent protein kinase
The invention provides a nucleotide sequence for preventing toxoplasma infection. The nucleotide sequences selected from the followings are contained: 1) 724-1470th nucleotide sequences as shown in SEQ ID No.3, and / or 724-2472nd nucleotide sequences as shown in SEQ ID No.1; 2) nucleotide sequences complementary with the nucleotide sequences as shown in 1); and 3) nucleotide sequences which perform substituting, deletion and addition modification on one or a plurality of basic groups of the nucleotide sequences as shown in 1) or 2). The pVAX-CDPK (Calcium Dependent Protein Kinase)1 can effectively prevent and control the infection of a toxoplasma animal model (Kunming mice), and pVAX-IL21-IL15 not only can be independently used for effectively preventing and controlling the toxoplasma infection, but also can be used as a cytokines adjuvant for effectively improving the immune effect of a vaccine pVAX-CDPK1.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Application of lncRNA 11496 in diagnosis and treatment of toxoplasmosis
ActiveCN109929939AVerify impactThe value of good practical applicationMicrobiological testing/measurementAntiparasitic agentsMedicineBiological target
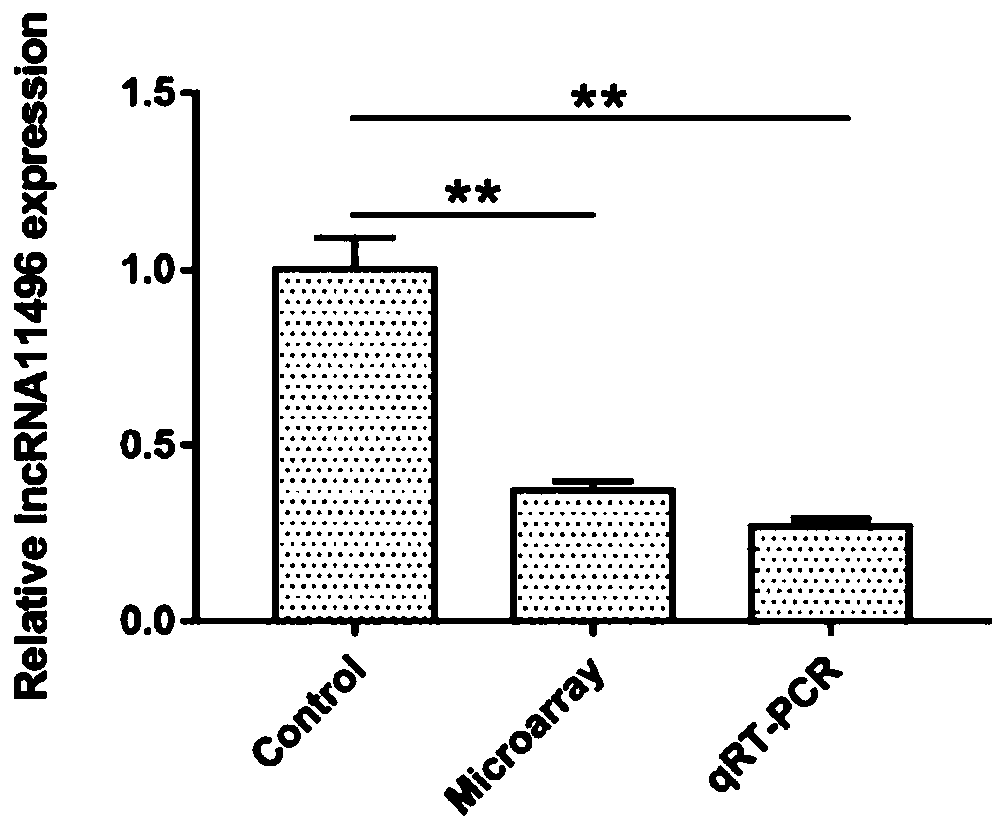
The invention provides application of lncRNA 11496 in diagnosis and treatment of toxoplasmosis and belongs to the technical field of biological medicine and molecular biology. It is found through research that NONMMUG011496 (lncRNA 11496) was significantly down-regulated in the brain tissue of mice infected with Toxoplasma gondii; meanwhile, the expression of lncRNA 11496 in brain tissues of micechronically infected with toxoplasma gondii affected the proliferation, differentiation and apoptosis of nerve cells, which was closely related to Toxoplasma gondii infection. The lncRNA 11496 is a noncoding RNA first found in brain tissues chronically infected with toxoplasma gondii. Therefore, the lncRNA 11496 can serve as an important biological target, provides a basis for diagnosis of toxoplasma encephalopathy and research and development of targeted anti-toxoplasma drugs based on the lncRNA 11496, and has a good practical application value.
Owner:SHANDONG UNIV
Agentia for preventing toxoplasma infection and application
ActiveCN104800843AImprove immunityProtozoa antigen ingredientsAntiparasitic agentsAgonistTGE VACCINE
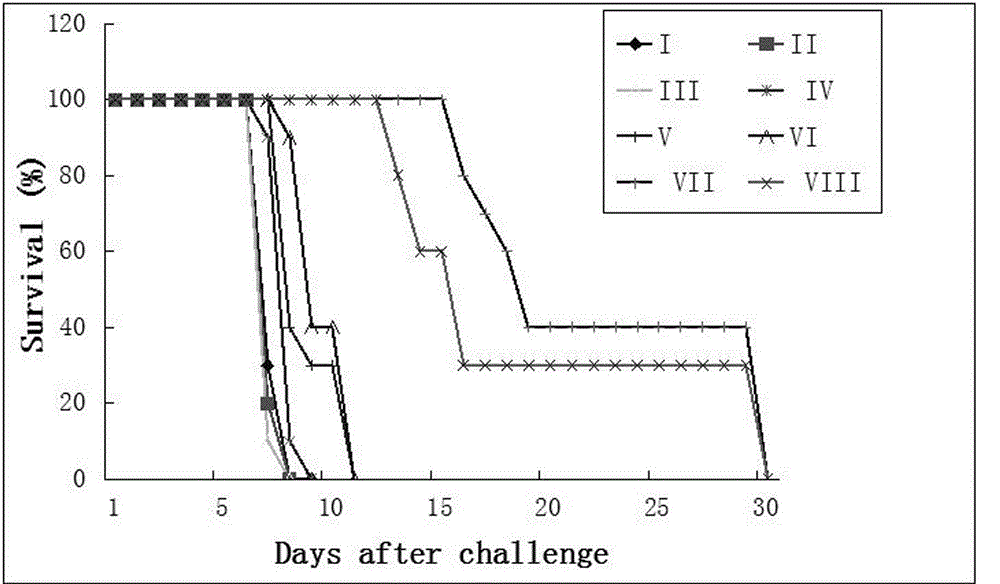
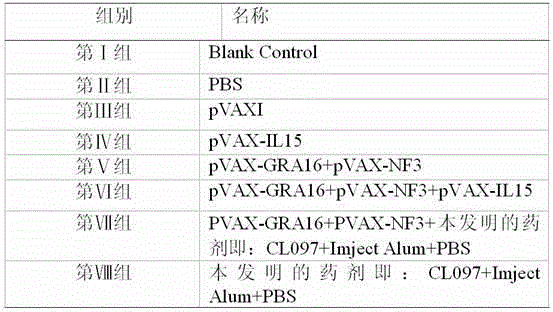
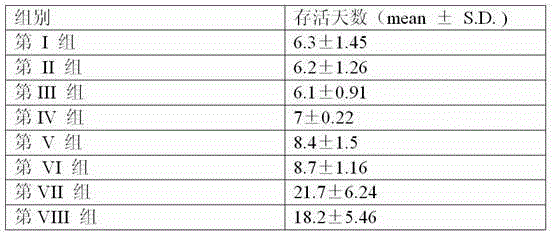
The invention provides agentia for preventing toxoplasma infection. The agentia is prepared in a way that aluminium hydroxide adjuvant adsorbs TLR7 / 8 agonist CL 097. The agentia, as immunologic adjuvant, can enhance the immune effect of toxoplasma vaccine and can also serve as toxoplasma vaccine independently.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Methods and compositions of protein antigens for the diagnosis and treatment of toxoplasma gondii infections and toxoplasmosis
InactiveCN103517714AMonitoring and Analyzing Treatment EfficacyThe goal is obviousProtozoa antigen ingredientsAntiparasitic agentsDiseaseDiagnostic test
Contemplated compositions, devices, and methods are drawn to various antigens from the pathogen T. gondii and their use in various diagnostic tests, vaccines, and therapeutic agents. In particularly preferred aspects, the antigens are immunodominant and have quantified and known relative reactivities with respect to sera of a population infected with the pathogen, and / or have a known association with a disease parameter.
Owner:英珀特疗法有限公司 +1
Indirect ELISA (enzyme-linked immuno sorbent assay) kit for detecting human toxoplasma gondii antibody and preparation method of indirect ELISA kit
InactiveCN108732360ARich genetic diversityImproving immunogenicityBiological material analysisBiological testingPositive controlSorbent
The invention discloses an indirect ELISA (enzyme-linked immuno sorbent assay) kit for detecting a human toxoplasma gondii antibody and a preparation method of the indirect ELISA kit, and belongs to the field of biotechnology. The kit comprises a recombinant antigen GRA15 pre-coated plate, a sample diluent, a positive control, a negative control, an enzyme conjugate, a blocking solution, a washingsolution, a color developing solution and a terminating solution, wherein the amino acid sequence of a recombinant antigen GRA15 is shown in SEQ ID NO: 1. Compared with the existing detection technology, the indirect ELISA detection method has the advantages of being simple to operate, good in repeatability, economic and suitable for large-scale sample detection. Toxoplasma gondii Chineses1 strain GRA15 protein isolated in China is used as the coating antigen of the indirect ELISA method, and the kit has higher specificity for detection of toxoplasma gondii infection of Chinese people.
Owner:ANHUI MEDICAL UNIV
Identified mmu-miR-217-5p capable of detecting toxoplasma gondii infection
InactiveCN103866012AIncreased sensitivityImprove featuresMicrobiological testing/measurementDNA/RNA fragmentationGondii toxoplasmaComplementary deoxyribonucleic acid
The invention discloses identified mmu-miR-217-5p capable of detecting toxoplasma gondii infection. In a toxoplasma gondii infected mouse model, cDNA (complementary deoxyribonucleic acid) is obtained by extracting general RNA (ribonucleic acid) of plasma and inversely transcribing; high-expression mmu-miR-217-5p is screened by adopting a Q-PCR (quantitative polymerase chain reaction) technology, is remarkably related to toxoplasma gondii infection, and can be used as identified miRNA for toxoplasmosis diagnosis. The mmu-miR-217-5p is used as a marker to establish a Q-PCR detection method of toxoplasma gondii infection, has relatively high sensitivity and specificity, provides a reliable detection basis and quick detection method to clinical diagnosis for toxoplasmosis, and has an important clinical application value.
Owner:JILIN UNIV
Multivalent adenovirus vaccine for preventing toxoplasmosis
ActiveCN104117072AAvoid infectionControl Cyst FormationGenetic material ingredientsAntiparasitic agentsAntigenDigestion
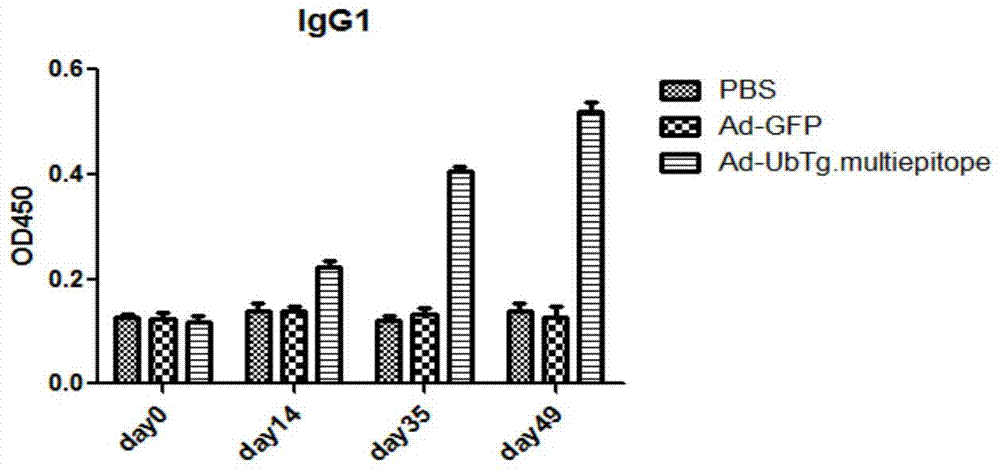
The invention discloses a compound multivalent antigen gene, a recombinant plasmid and an adenovirus vaccine for preventing toxoplasmosis. The preparation process comprises the following steps: connecting a ubiquitin protein gene, a toxoplasmosis tachyzoite antigen gene, a bradyzoite antigen gene, a sporozoite antigen SPA gene and an AS15 gene, and synthetizing the compound multivalent antigen gene for preventing toxoplasmosis; carrying out double digestion on the synthesized target gene and then inserting the synthesized target gene between BamHI and SmaI of a vector to generate the recombinant plasmid; and carrying out digestion, PCR and sequencing identification on the recombinant plasmid, extracting plasmid and purifying; transferring the recombinant plasmid and skeleton plasmid into cells together, packing and removing toxins, so as to obtain the recombinant adenovirus vaccine. By adopting the adenovirus vaccine disclosed by the invention, toxoplasma infection of people or animals can be effectively prevented, formation of brain tissue cysts in the chronic infection process of toxoplasma is controlled, the immunologic reaction and the survival rate are improved in acute infection, and candidate components of an effective vaccine with comprehensive protection are provided.
Owner:SHANDONG UNIV
Immune vaccine for preventing toxoplasma gondii infection
ActiveCN105169387AProlong survival timeReduce the number of cysts in the brainProtozoa antigen ingredientsBacteriaBALB/cImmune effects
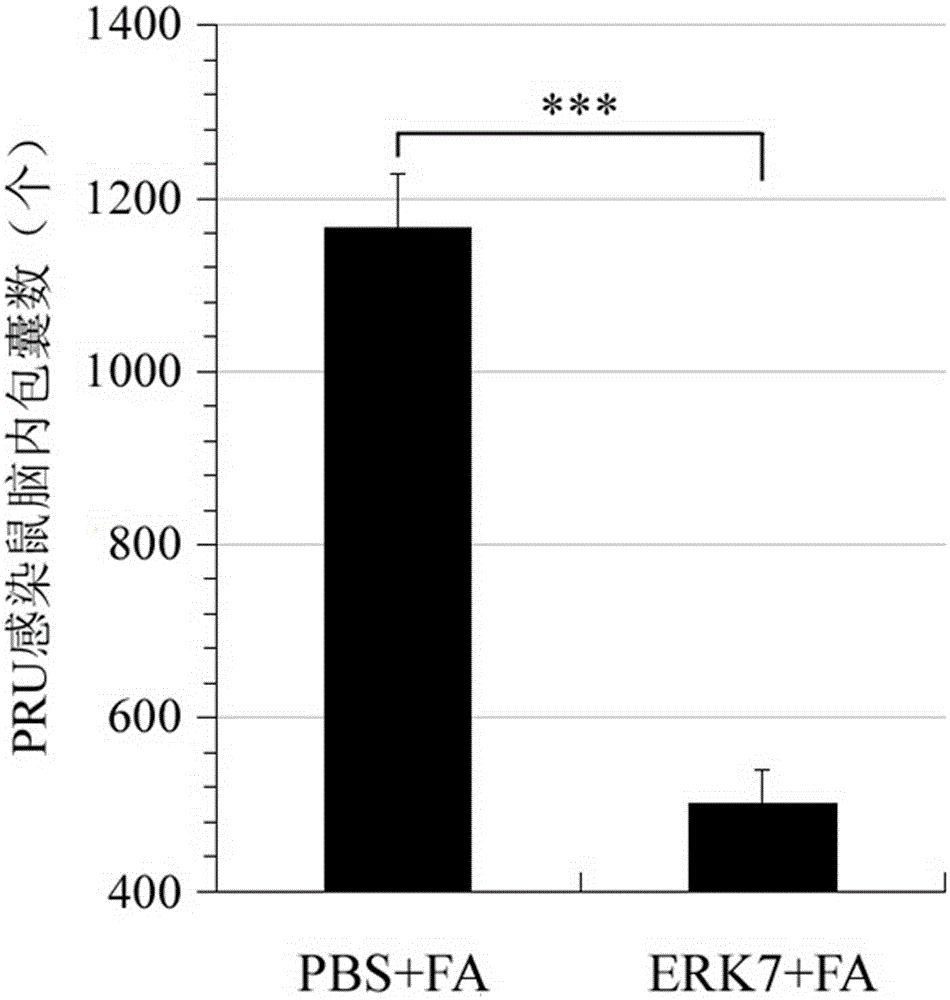
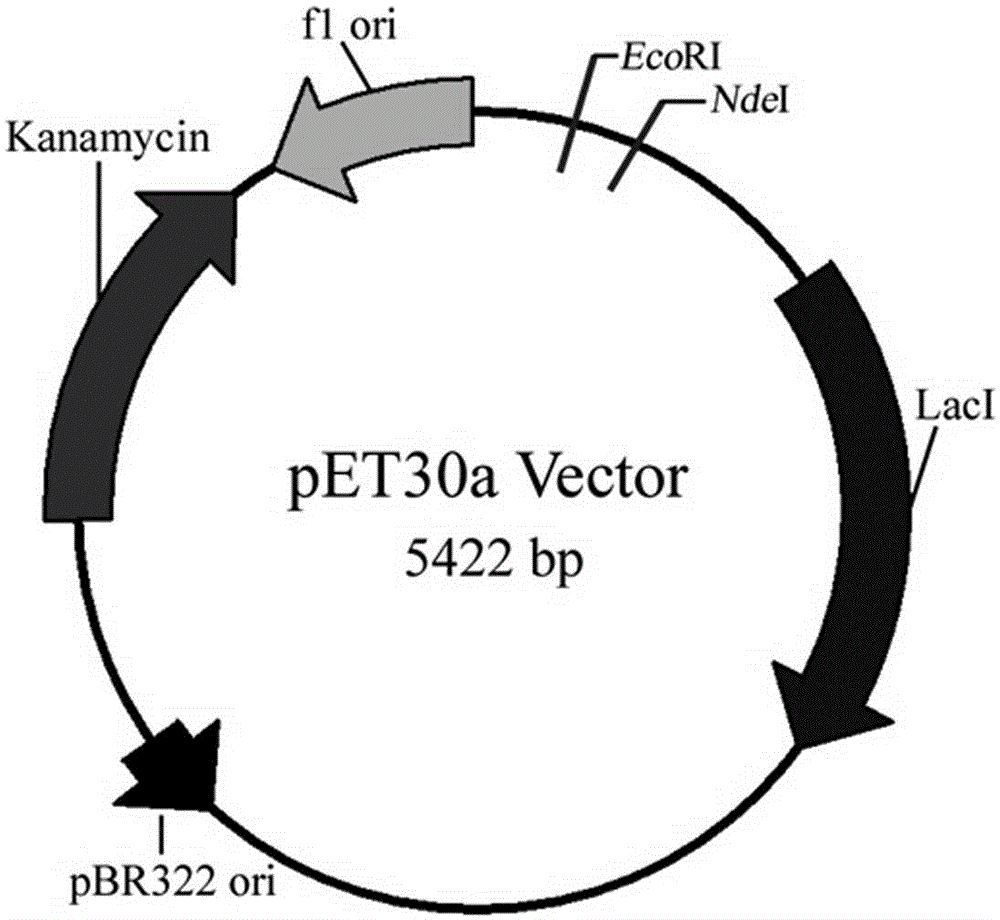
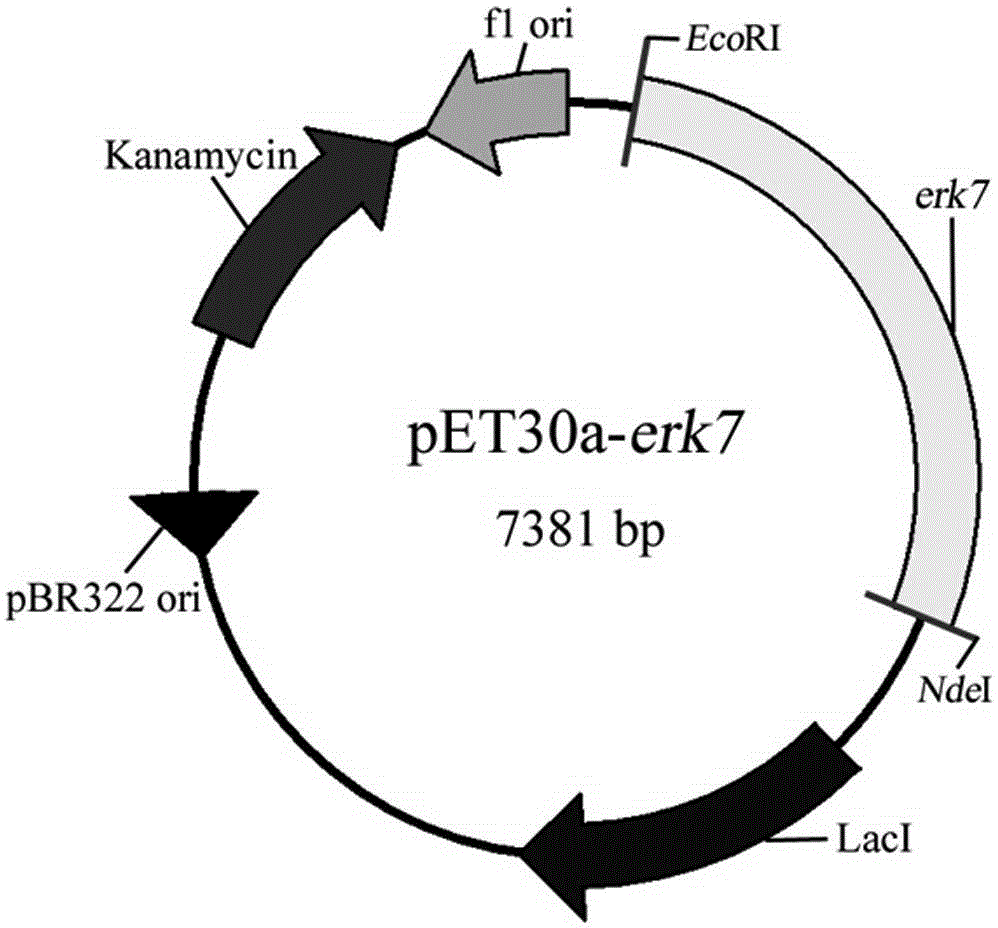
The invention provides an immunogenicity composition which comprises erk7 recombinant protein and adjuvant. The invention further provides an immune vaccine for preventing toxoplasma gondii infection. The effective component of the immune vaccine is optional one of the immunogenicity compositions in claims 1-4. The immune vaccine has the advantages that the vaccine can effectively prolong the survival time of toxoplasma gondii GT1 acute infection BALB / c mice, the vaccine has no evident effect on PRU acute infection but can greatly reduce brain cyst number in toxoplasma gondii PRU acute infection, and a good immune effect is achieved.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
RH: delta NPT1 live attenuated vaccine for preventing toxoplasma infection and preparation method and application thereof
InactiveCN109999188AGood immune protectionDecreased number of cysts in the brainProtozoa antigen ingredientsVectorsGondii toxoplasmaCell culture media
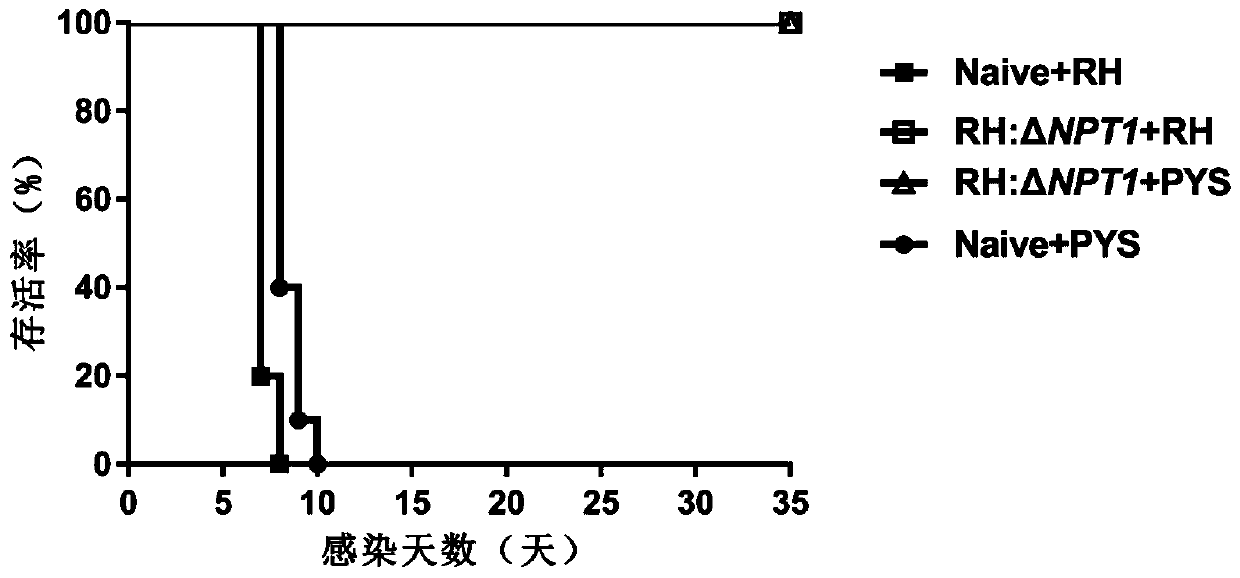
The invention discloses a RH: deltaNPT1 live attenuated vaccine for preventing toxoplasma infection. The method comprises the steps that plasmid pSAG1::Cas9::U6sgNPT1 and DHFR resistant fragments areconstructed, a theoxoplasma gondii RH: delta NPT1 strain is constructed, and a tachyzoite suspension of toxoplasma gondii Attenuated strain RH: delta NPT1 is prepared in a cell culture medium using PBS as a solvent. The method establishes an immunization dosage form of the live attenuated vaccine, and proves the immunization procedure and dosage thereof. The animal experiment shows that the live attenuated vaccine prepared by the method has a strong immunoprotective effect on acute toxoplasma gondii infection or chronic toxoplasma gondii infection, can be applied to the immune protection of the acute toxoplasma infection or the chronic toxoplasma infection, and has a great application value in the prevention and control of toxoplasma gondii.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
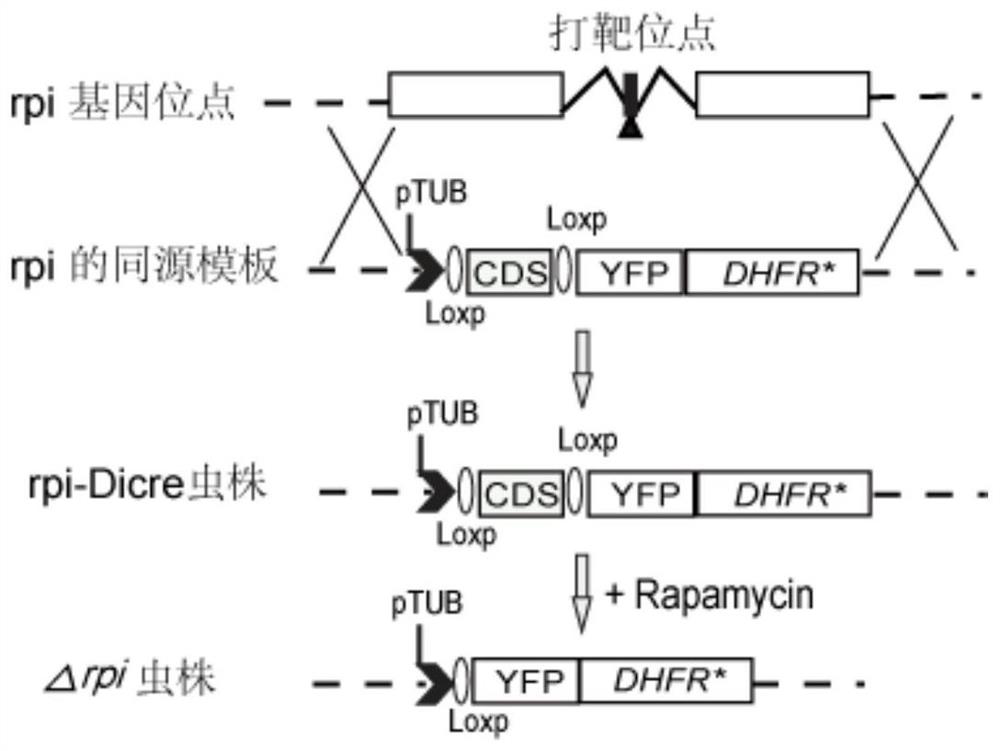
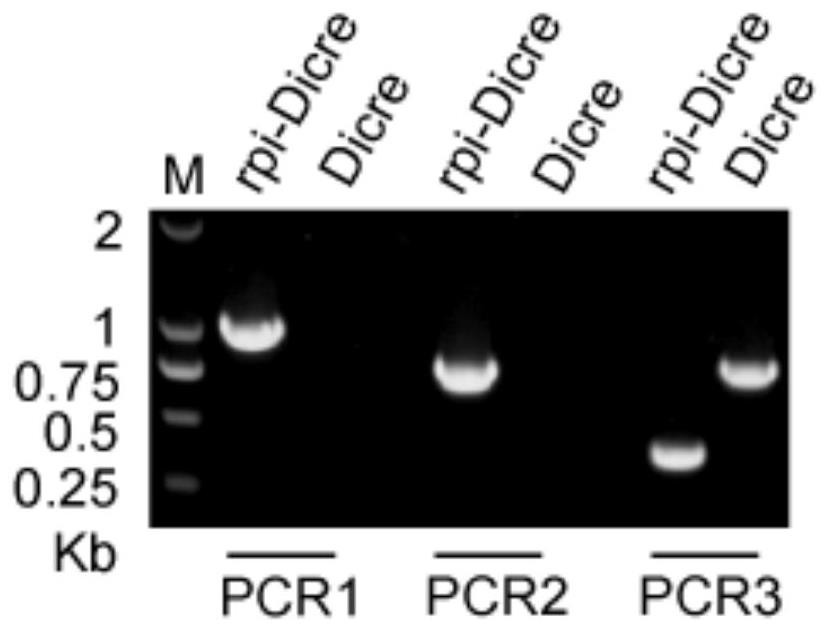
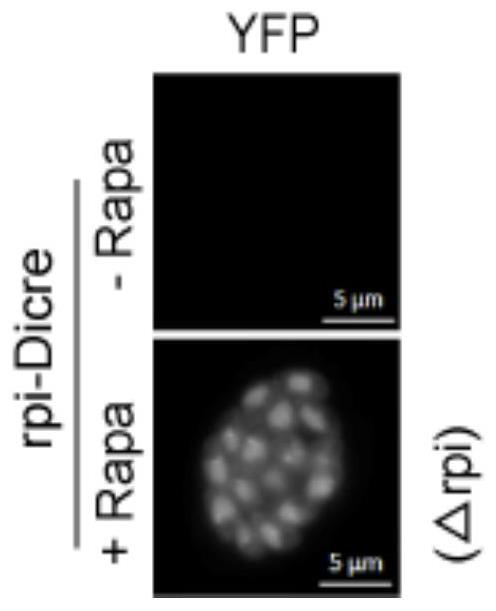
Toxoplasma gondii ribulose-5-phosphate isomerase TgRPI gene edited strain and application thereof
The invention relates to a toxoplasma gondii ribulose-5-phosphate isomerase TgRPI gene edited strain and an application thereof. The invention discloses a potential vaccine and drug design target and a gene knockout strain for preventing toxoplasma gondii infection. The design target of the vaccine and the drug is a toxoplasma gondii ribulose-5-phosphate isomerase TgRPI gene, and the nucleotide sequence of the toxoplasma gondii ribulose-5-phosphate isomerase TgRPI gene is as shown in SEQ ID NO: 2. The TgRPI gene is knocked out of the gene knockout strain, the growth speed of the gene knockout strain in vitro is slowed down, but the gene knockout strain can be subcultured, and the gene knockout strain has the characteristics of small toxicity, less reproduction and the like in animal bodies, and can improve the immunocompetence of animals to toxoplasma gondii. The TgRPI plays an important role in in-vivo and in-vitro growth of toxoplasma gondii, so that the TgRPI has the potential of becoming a vaccine and a drug target, and the gene knockout strain has the potential of becoming an anti-toxoplasma gondii genetic engineering vaccine.
Owner:SOUTH CHINA AGRI UNIV +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com