Nucleotide sequence for preventing sheep toxoplasmosis infection, vector, protein, vaccine, preparation method and application thereof
A nucleotide sequence, Toxoplasma gondii technology, applied in the fields of immunology and biology, can solve the problems of inability to widely use attenuated vaccines, reversion of virulence, insufficient attenuation, etc., and achieve strong cellular immunity and humoral immunity. The effect of preventing toxoplasmosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
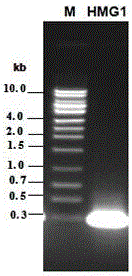
[0032] Embodiment 1: Construction of the prokaryotic expression vector of Toxoplasma gondii recombinant protein
[0033] According to the open reading frame of the Toxoplasma gondii HMG1 gene sequence and the physical map of the prokaryotic expression vector pET28a, primers were designed and enzyme cutting sites were introduced.
[0034] Upstream: G GAATTC GCACCGAAGAAGGTGACAAAGAAG
[0035] Downstream: CC CTCGAG TTTCTTCTTGTTGTAGAGAGAG
[0036] Use the RNA extraction kit to extract the total RNA of Toxoplasma trophozoites, and use the RT-PCR kit to amplify the HMG1 gene (the general RT parameters are: 37°C for 60min; 65°C for 20min; 4°C for 5min. The PCR parameters are: 95°C for 3min; 94°C 1min; 59.5°C for 40s, 72°C for 1min, and after 30 cycles of extension at 72°C for 10min), the PCR purified product was cloned into pGEM-T vectorsystem. Positive clones were identified by enzyme digestion and sequencing, and the positive plasmids were double-enzymeed by EcoRI and XhoI Aft...
Embodiment 2
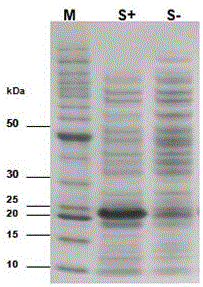
[0037] Embodiment 2: Purification of expressed protein
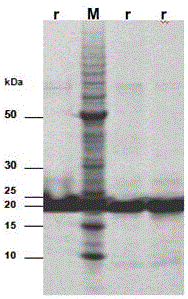
[0038] (1) Extraction and solubility verification of expressed protein
[0039] A single colony of E.coli BL21(DE3) transformed with the recombinant plasmid pET-28a(+)-HMG1 was selected as the starting strain, inoculated in 8 mL LB liquid medium, and cultured overnight at 37°C with shaking. The next day, the seed solution was transferred to 400mL LB liquid medium for multiplication at 37°C, and the expression was induced under optimal conditions when the OD600 of the bacterial solution was monitored to 0.6-0.7. Collect the bacteria by centrifugation, suspend the bacteria with 10mL soluble protein lysate, add 100μL 0.1M PMSF, freeze and thaw 3 times (-80℃, 1h / 37℃, 10min), after sonication, or add 100μL DNase and 100μL 100× DNase Buffer and 100μL RNase, in a water bath at 37°C for 15 minutes, centrifuge to get the supernatant, which is the soluble protein (active protein). Suspend the precipitate with 10 mL of insoluble ...
Embodiment 3
[0042] Example 3: Detection of the immunogenicity of expression products to mouse macrophages
[0043] RAW264.7 cells were seeded on 24-well culture plates at 0.5x106 / mL, 1 mL per well, 5% CO2, and cultured at 37°C for 24 hours. After aspirating the supernatant, dissolve the prepared Toxoplasma gondii recombinant HMG1 protein in the RAW264.7 cell culture medium, and add it to the cell culture plate at a concentration of 1 μg / mL, 0.1 μg / mL, and 0.01 μg / mL at 1 mL / well In the experiment, an equal volume of blank plasmid-purified protein NR was used as a negative control. The cell culture plate was placed in 5% CO2, cultured in a 37°C incubator for 48 hours, the supernatant was collected, and the level of TNF-a was detected by ELISA experiment. The results show that the production of TNF-a is different, and the amount of TNF-a increases with the increase of protein concentration, ranging from 180pg-520pg, the results are shown in the appendix Figure 4 ; The results show that t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com