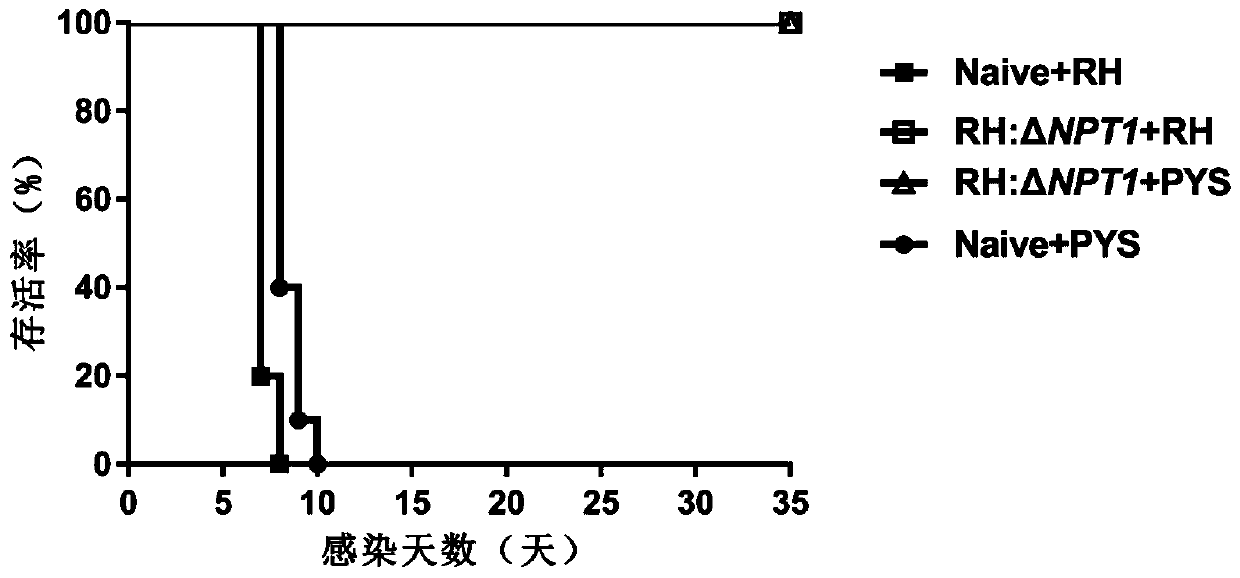
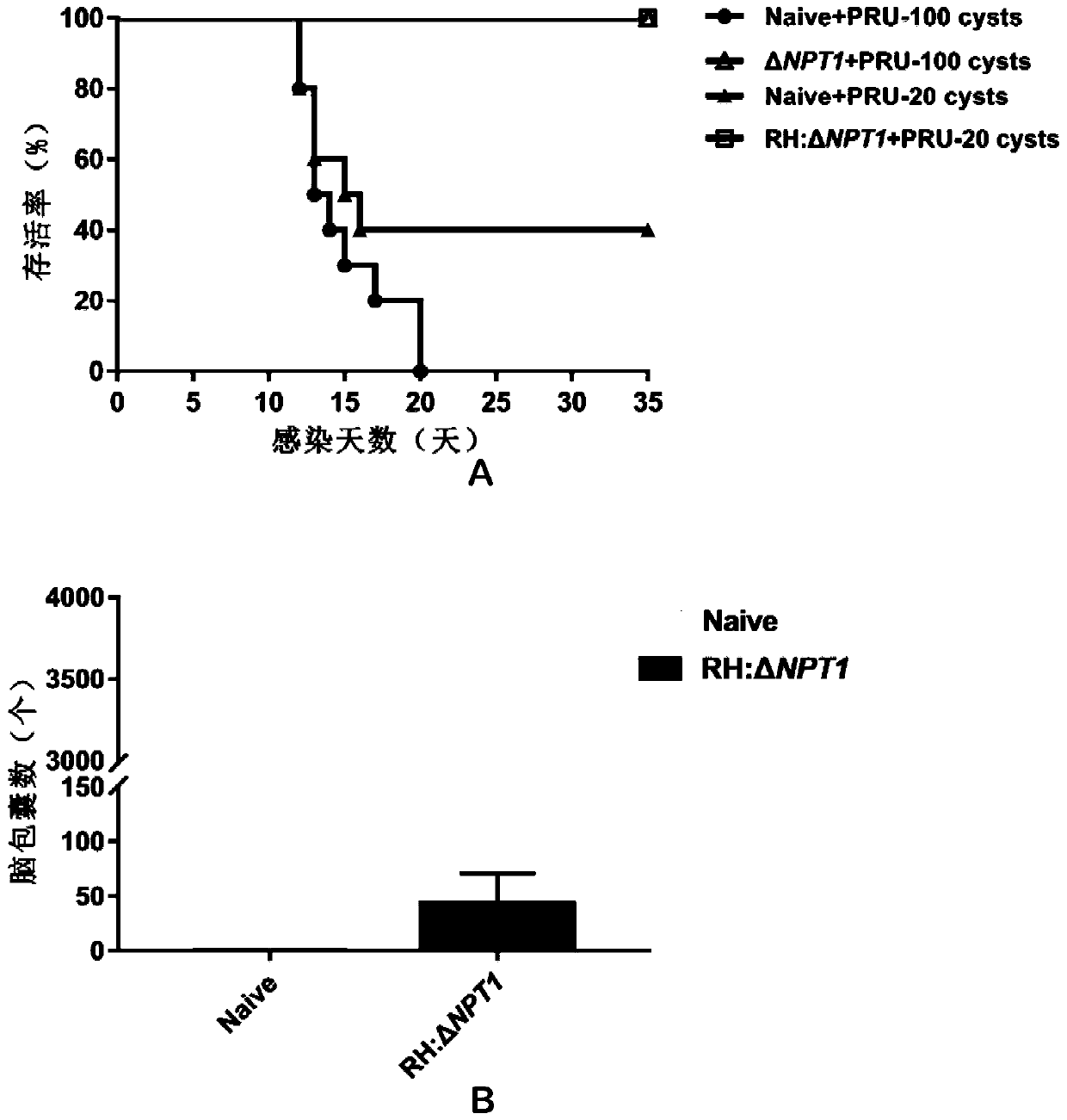
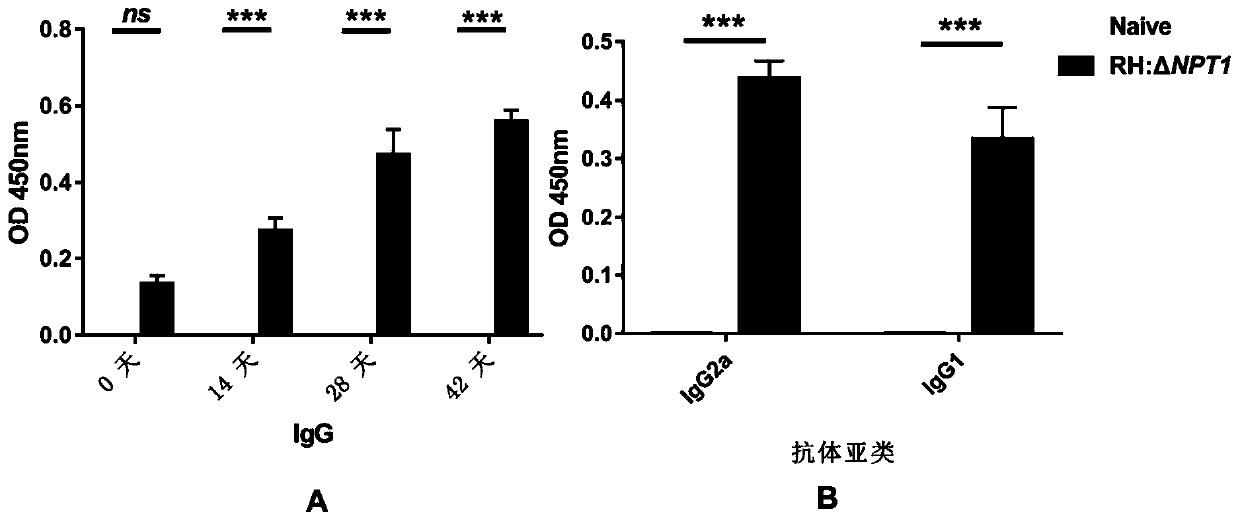
RH: delta NPT1 live attenuated vaccine for preventing toxoplasma infection and preparation method and application thereof
An attenuated live vaccine and Toxoplasma gondii technology, applied in botany equipment and methods, biochemical equipment and methods, and other methods of inserting foreign genetic materials, etc., can solve the problem of inability to achieve complete protection and unsatisfactory immune protection effect , large application limitations and other problems, to achieve good immune protection effect, reduce the number of brain cysts, and achieve the effect of great application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0028] In order to make the object, technical solution and advantages of the present invention clearer, the present invention will be further described in detail below in conjunction with the accompanying drawings and embodiments. It should be understood that the specific embodiments described here are only used to explain the present invention, not to limit the present invention.
[0029] 1. Construction of knockout plasmids
[0030] Design the sgRNA according to the NPT1 gene (TGME49_215490) in the TOXODB of the Toxoplasma genome website: GATGAAGGTGCAAGCTCCCG, and then use the Q5 site-directed mutagenesis kit to replace the sgRNA in pSAG1::Cas9::U6sgUPRT with NTP1 to construct the plasmid pSAG1::Cas9::U6sgNPT1 . The DHFR-resistant fragment was amplified from the plasmid pUPRT-DHFR-D by primers, and the primers used were DHFR-F: AAGCTTCGCCAGGCTGTAAA and DHFR-R: GGAATTCATCCTGCAAGTGC.
[0031] 2. Construction of Toxoplasma gondii RH:ΔNPT1 strain
[0032] Inoculate HFF cells ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com