Multivalent adenovirus vaccine for preventing toxoplasmosis
A technology for toxoplasmosis and adenovirus, applied in the field of adenovirus vaccines, can solve problems such as the difficulty of vaccine protection, and achieve the effects of improving immune response and survival rate, reducing the formation of cysts in the brain, and prolonging survival time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1: Construction of Toxoplasma gondii polyvalent adenovirus vaccine
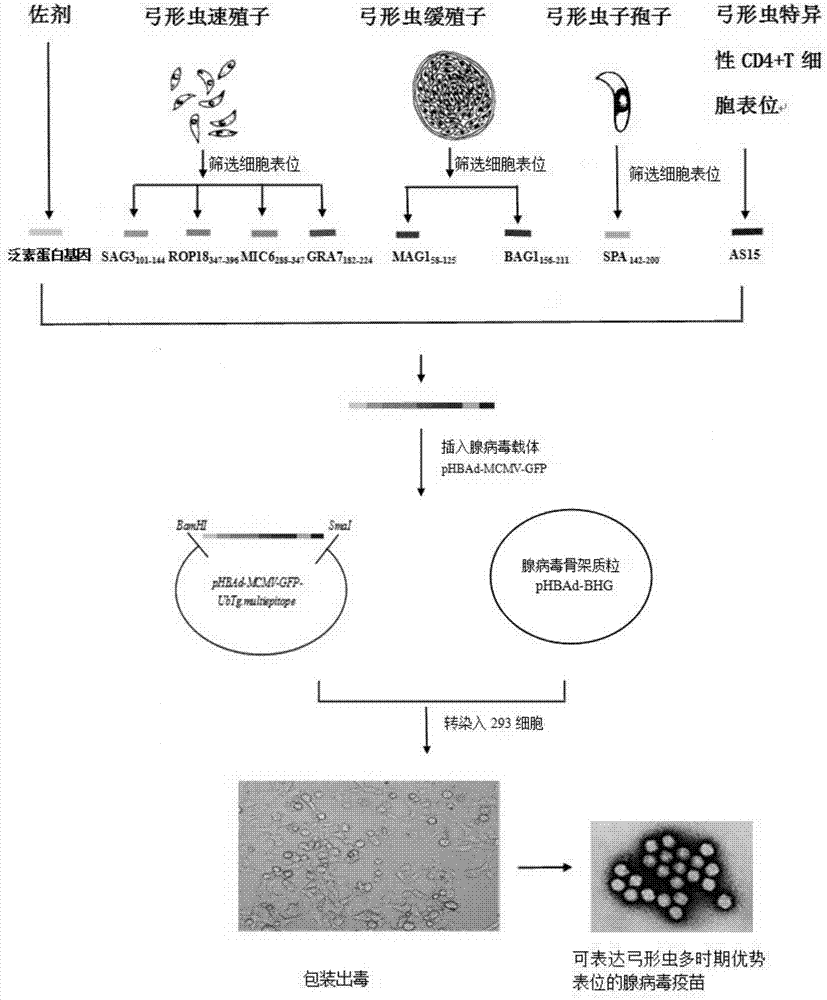
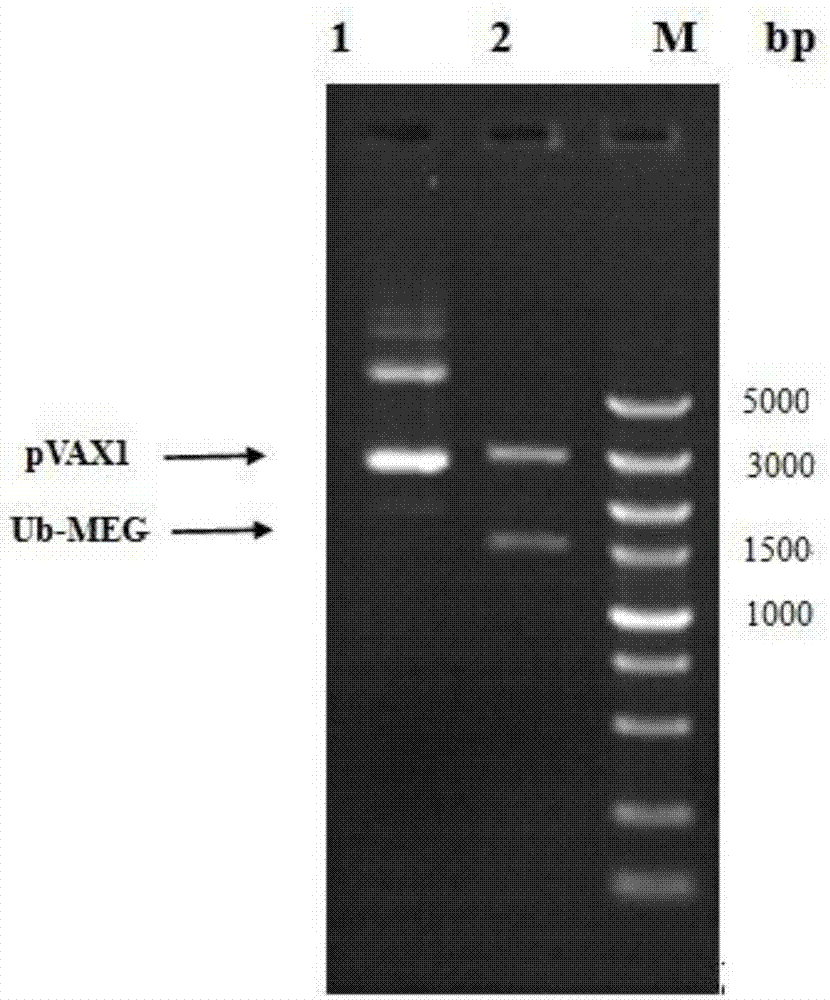
[0029] Such as figure 1 As shown, first from the Toxoplasma gondii tachyzoite antigens SAG3, ROP18, MIC6, GRA7, bradyzoite antigens MAG1, BAG1 and sporozoite antigen SPA genes, IEDB software was used to screen out epitopes that are concentrated and have both human and BALB / C mouse MHC molecularly restricted gene segment: SAG3 101-144 ,ROP18 347-396 ,MIC6 288-347 ,GRA7 182-224 ,MAG158-125 ,BAG1 156-211 ,SPA 142-200 and a proven effective CD4 + The T cell epitope AS15 gene fragment constitutes a linear compound multivalent antigen gene, and its N-terminus is linked to the ubiquitin protein gene, which is synthesized by Suzhou Jinweizhi Biotechnology Co., Ltd.
[0030] Among them, SAG3 101-144 The nucleotide sequence of the gene fragment is: ggcttgggcg gagagttttt gccgctcgaa ggcggcacgt cgtcgtaccc gcgagtatgt cacattgatg ccaaggacaa gggcgactgc gagcgcaaca agggctttct gaccgactac ataccgggcg cg, as...
Embodiment 2
[0041] Embodiment 2: vaccine immunization BALB / c mice
[0042] SPF grade female BALB / c mice (6-8 weeks) were purchased from the Experimental Animal Center of Shandong University. 40 mice were randomly divided into 6 groups, and each group of mice in the experimental group was intramuscularly injected with 3x10 8PFU, the adenovirus vaccine Ad-UbTgMEG that the embodiment of the present invention 1 prepares, the control group injects immunization 3x10 8 PFU empty virus Ad-GFP and 100 μl PBS. Mice were immunized twice with an interval of three weeks.
Embodiment 3
[0043] Embodiment 3: the mensuration of immune mouse humoral immunity level
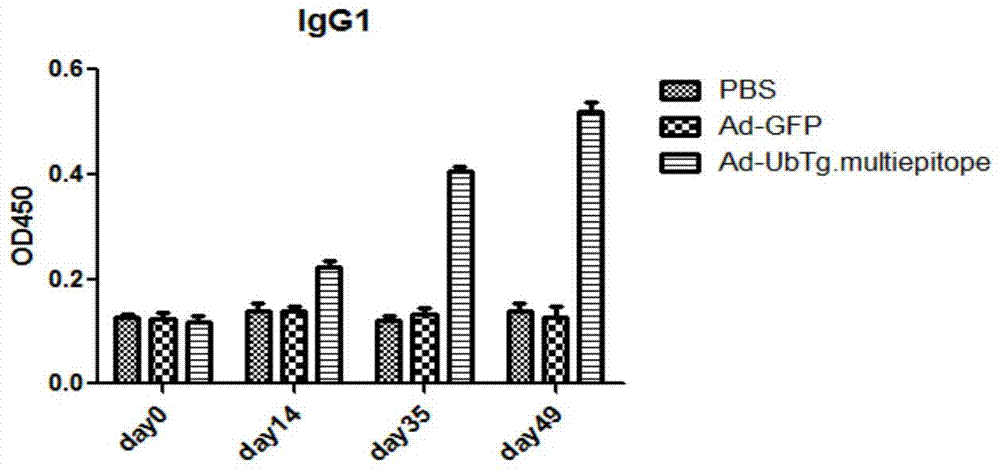
[0044] On the 0th, 14th, 35th, and 49th days, the blood was collected from the inner canthus of the mice. The blood samples were first allowed to stand at room temperature for 3 hours, and then centrifuged at 3000 rpm for 30 minutes to collect serum. Serum levels of total anti-T. gondii immunoglobulins (IgG1 and IgG2a) were determined using enzyme-linked immunosorbent assay. The result is as Figure 3a to Figure 3b shown. After two immunizations, compared with the control group, PBS and Ad-GFP groups, the antibody titer level of the vaccine immunization group increased significantly. On the 35th day and 49th day, the mice in the Ad-UbTg. High levels of Toxoplasma-specific IgG antibodies were detected (P<0.01).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com