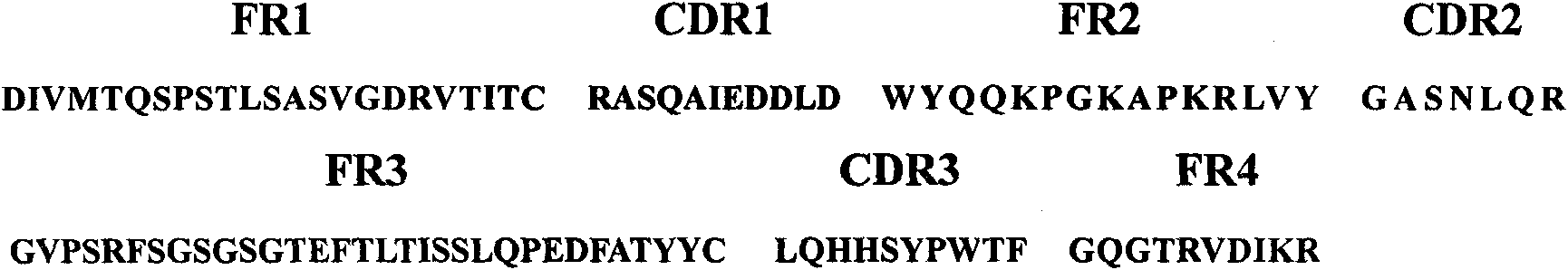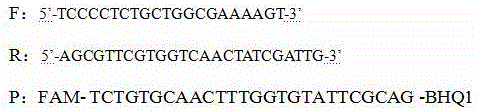Patents
Literature
150 results about "Toxoplasmas" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Electrochemical immunosensor for detecting toxoplasma gondii IgM antibody and preparation method thereof
InactiveCN102914648AImprove conductivityEasy to passMaterial electrochemical variablesMedical diagnosisElectronics
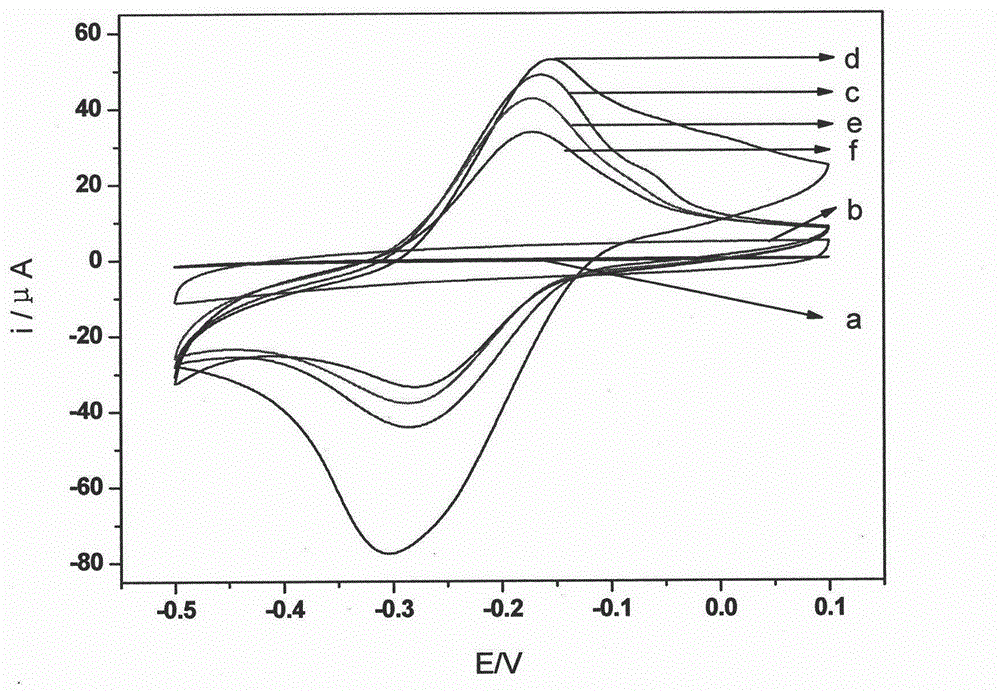
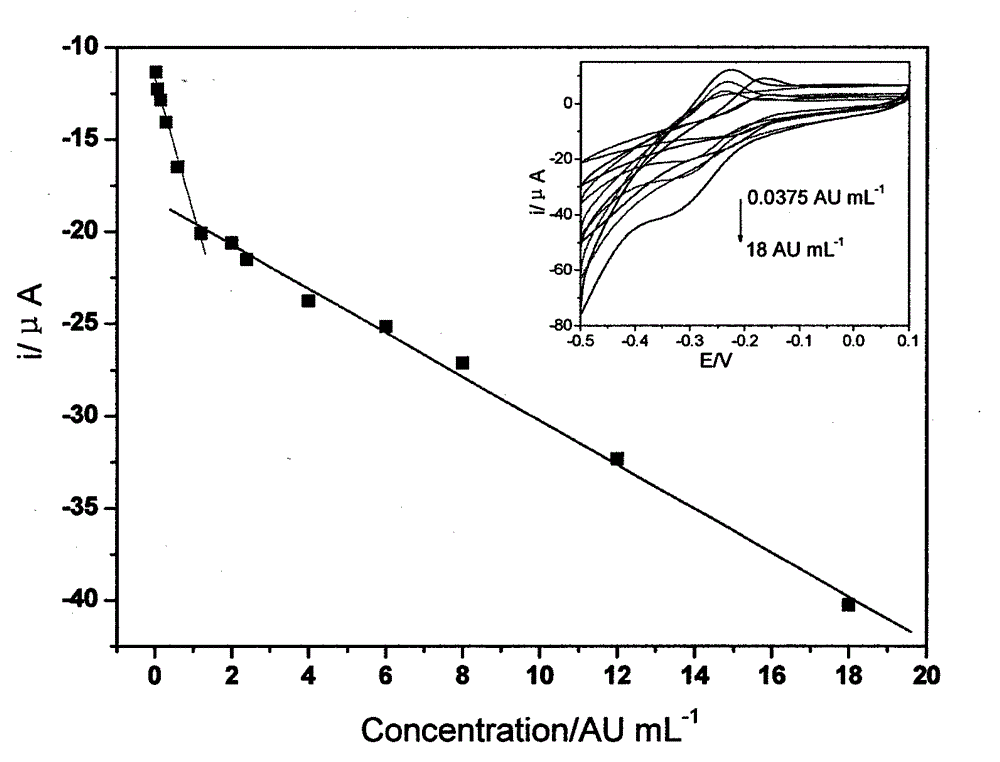
The invention belongs to the technical field of analytical chemistry and chemical sensors and discloses an electrochemical immunosensor for detecting a toxoplasma gondii IgM (Immunoglobulin m) antibody (Tg-IgM) of a gravida and a preparation method of the electrochemical immunosensor. The immunosensor is prepared by sequentially modifying graphene, polythionine, gold nanoparticles and capture antigen to the surface of a glassy carbon electrode. An enzyme-functionalized nano-composite detection probe with an electrical signal amplifying function is prepared by assembling enzyme and a second antibody with high proportions on an Au-Fe3O4 surface. According to the sandwich immunoassay principle, the concentration of Tg-IgM is determined by using an electrochemical signal generated by catalysis of enzyme to a substrate. According to the electrochemical immunosensor, the specificity of immunoreaction is combined with the sensitivity of electrochemical detection; the transmission of electronics is promoted by using the graphene, the polythionine, the gold nanoparticles, Au-Fe3O4 and other material; and the sensitivity of the detection is improved. The electrochemical immunosensor has the advantages of simplicity and convenience for operation, favorable regeneration performance and detection cost reduction. The electrochemical immunosensor prepared on the basis can be also used for detecting other immunological markers and has favorable application prospect in medical diagnosis.
Owner:CHONGQING MEDICAL UNIVERSITY
Monoclonal antibody of toxoplasma gondii resistant MIC3 protein and application monoclonal antibody
ActiveCN103333864AHigh potencyStrong specificityTissue cultureImmunoglobulinsHybridoma cellToxoplasma gondii
The invention provides a monoclonal antibody of a toxoplasma gondii resistant MIC3 protein and an application of the monoclonal antibody, and relates to the technical field of biology. The preservation number of a hybridoma cell strain D3 of secreting the monoclonal antibody of the toxoplasma gondii resistant MIC3 protein is CCTCCC (China Center For Type Culture Collection) 201383. The invention also discloses the monoclonal antibody of the toxoplasma gondii resistant MIC3 protein secreted by the hybridoma cell strain D3, and an application of the monoclonal antibody of the toxoplasma gondii resistant MIC3 protein in preparation of a kit and a colloidal gold test strip for detecting the toxoplasma gondii. The monoclonal antibody of the toxoplasma gondii resistant MIC3 protein can be stably and efficiently secreted by the hybridoma cell strain D3; and the monoclonal antibody has high titer and good specificity on the toxoplasma gondii MIC 3 protein. Therefore, the monoclonal antibody can be used for preparing the kit and the colloidal gold test strip for detecting the toxoplasma gondii. The monoclonal antibody is simple in preparation method, simple in anti-body purification process, high in efficiency and low in cost.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Toxophasma gondii detecting kit based on recombined antigen
InactiveCN1861633AImprove immune activityExcellent repeatabilityBiological testingAnimals/human peptidesSephadexEscherichia coli
A reagent kit based on recombinant antigen for detecting Toxoplasma is prepared through taking 542-1218 fragment (t SAG1) from the primary surface antigen gene SAG1 of Toxoplasma, subcloning it to soluble expression carrier pET32a(+), transferring it to colibacillus, configuring engineering bacterium pET32a-tSAG1 / BL21, IPTG induced efficient expression, ultrasonic splitting to obtain supernatant, purifying by Ni-NTA and Sephadex-G75, and coating the microholes on ELISA plate. Its test paper can also be prepared by same way.
Owner:深圳市绿诗源生物技术有限公司
Screening and application method of small-molecule inhibitor capable of inhibiting proliferation of toxoplasma gondii
InactiveCN107884587APrevent proliferationR&D cost economyBiological testingScreening methodAccelerant
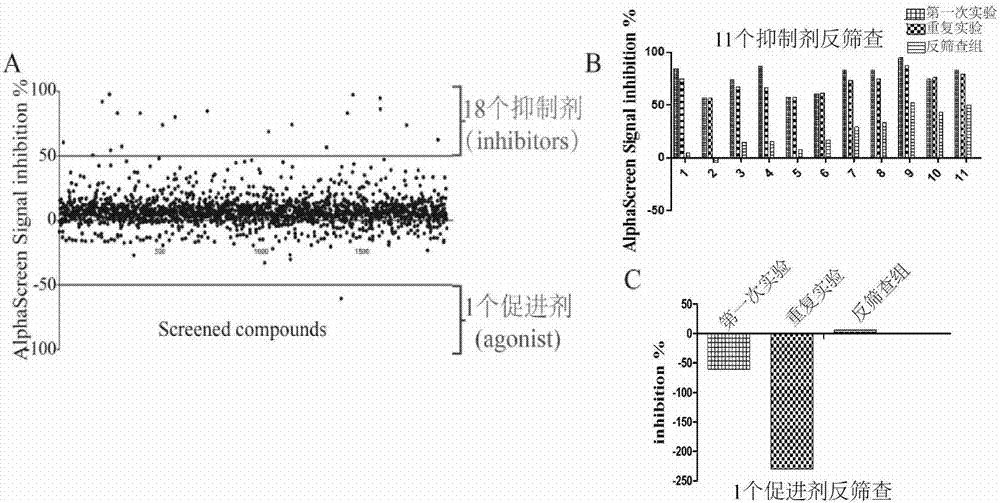
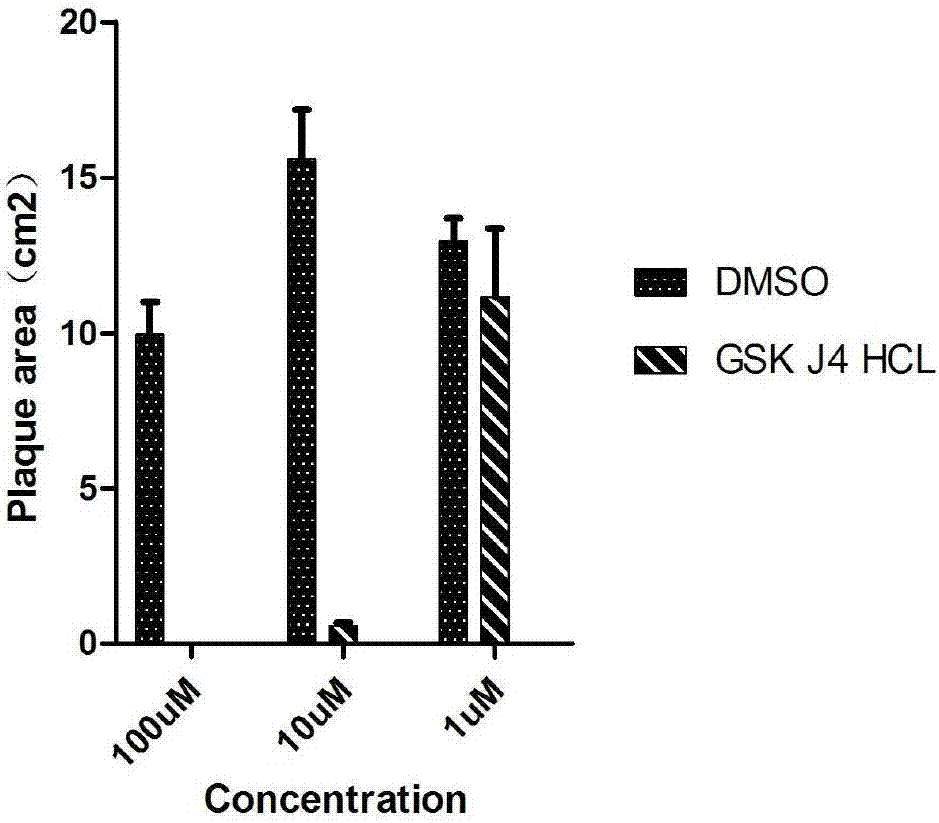
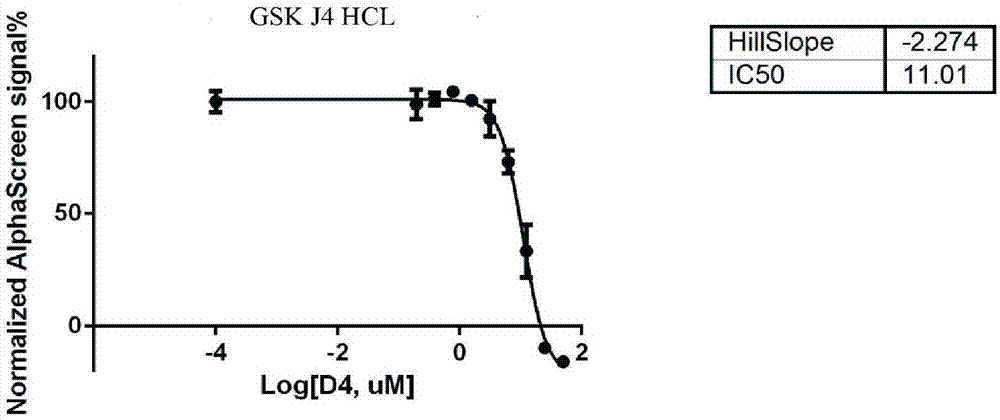
The invention discloses a screening method of small-molecule inhibitor capable of inhibiting proliferation of toxoplasma gondii. The method utilizes an Alphascreen experiment to primarily select 11 small-molecule inhibitors and one small-molecule accelerant, and the 12 drugs are used for clinical treatment. The 12 drugs are further screened at the cellular level, a plaque experiment proves that GSK J4HCL apparently inhibits the proliferation of toxoplasma gondii, and the value EC50 of the GSK J4HCL to toxoplasmagondii is determined to be about 4.5 muM by virtue of intracellular proliferation experiment. The AlphaScreen competitive inhibition experiment evaluates that the value IC50 of the GSK J4HCL for inhibiting the interaction of TgAtg8 to TgAtg3 is 11.01 muM. The CCK8 experiment is usedfor proving that the value IC50 of the drug for inhibiting the growth of a host cell is 34.359 muM which is far greater than the value EC50 of the drug for the insect, which illustrates that the GSKJ4HCL is a low-toxicity and high-efficiency drug for resisting the toxoplasma gondii.
Owner:WENZHOU MEDICAL UNIV
Real-time recombinase-mediated isothermal amplification nucleic acid kit for rapid detection of toxoplasma gondii and application thereof
InactiveCN111139309AHigh sensitivityStrong specificityMicrobiological testing/measurementMicroorganism based processesGondii toxoplasmaRecombinase
The invention discloses a real-time recombinase-mediated isothermal amplification nucleic acid kit for rapid detection of toxoplasma gondii and an application thereof. The kit comprises standards. Thestandards are a positive plasmid containing a 529 gene sequence, specific primers and probes designed for 529 gene of the toxoplasma gondii. The primers designed based on a specific conservative target sequence of the 529 gene of the toxoplasma gondii for qualitatively detecting the 529 gene of the toxoplasma gondii in tissues, feces or blood samples comprise an upstream primer and a downstream primer. According to the present invention, a real-time fluorescent RAA technology is used to establish the method for rapid detection of the toxoplasma gondii. Compared with fluorescent quantitative PCR, the method has the advantages of low cost, simpleness and fastness, can conduct detection at constant temperature of 36 DEG C within 20min, and directly read detection results by a portable instrument. The method of the present invention achieves minimum detection size for toxoplasma gondii genomes up to 102 copies, has sensitivity comparable to the traditional fluorescent quantitative PCR, and is suitable for rapid diagnosis of clinical samples and laboratories.
Owner:INST OF ANIMAL SCI OF CHINESE ACAD OF AGRI SCI
Arch insect circulating antigen immunity gold mark fast detecting reagent and method for producing the same
InactiveCN101271109ANo pollutionIncreased sensitivityMaterial analysisCellulosePolyclonal antibodies
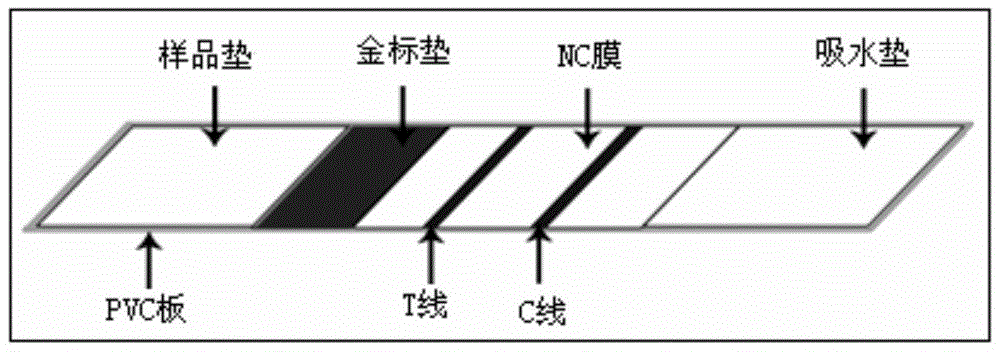
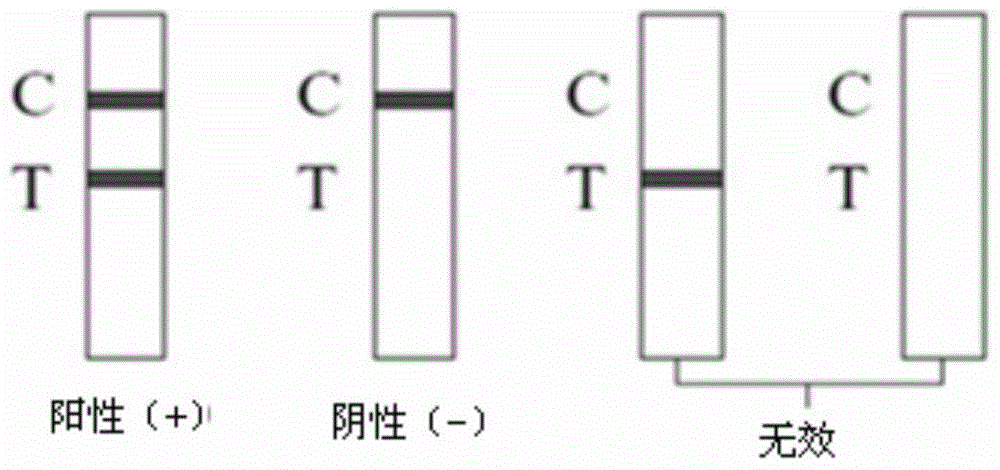
A rapid detection reagent of a toxoplasma circulating antigen immune colloidal gold marker comprises a sample pad, a bonding pad, a cellulose nitrate film, a water absorbent pad and a PVC back lining, wherein, one end of the PVC back lining is sequentially adhered with the sample pad, the bonding pad, the cellulose nitrate film and the water absorbent pad, the bonding pad is coated by a colloidal gold bonder with an anti-toxoplasma polyclonal antibody, the anti-toxoplasma polyclonal antibody and a toxoplasma metaboly secrete antigen are linearly coated on the cellulose nitrate film. The toxoplasma circulating antigen immune colloidal gold marker rapid detection reagent is prepared by preparing the anti-toxoplasma polyclonal antibody, preparing the toxoplasma metaboly secrete antigen, preparing the colloidal gold, preparing a colloidal gold marker of the anti-toxoplasma polyclonal antibody; the colloidal gold marker of the anti-toxoplasma polyclonal antibody is coated on the colloidal gold bonding pad, the anti-toxoplasma polyclonal antibody and the toxoplasma metaboly secrete antigen are linearly coated in a detection region and a stagnant control region of the cellulose nitrate film and the thorough drying and other processes are carried out. The rapid detection reagent of the toxoplasma circulating antigen immune colloidal gold marker can detect the toxoplasma circulating antigens of people and various animals, which has accurate sensitivity and simple operation, and being safe and stable.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
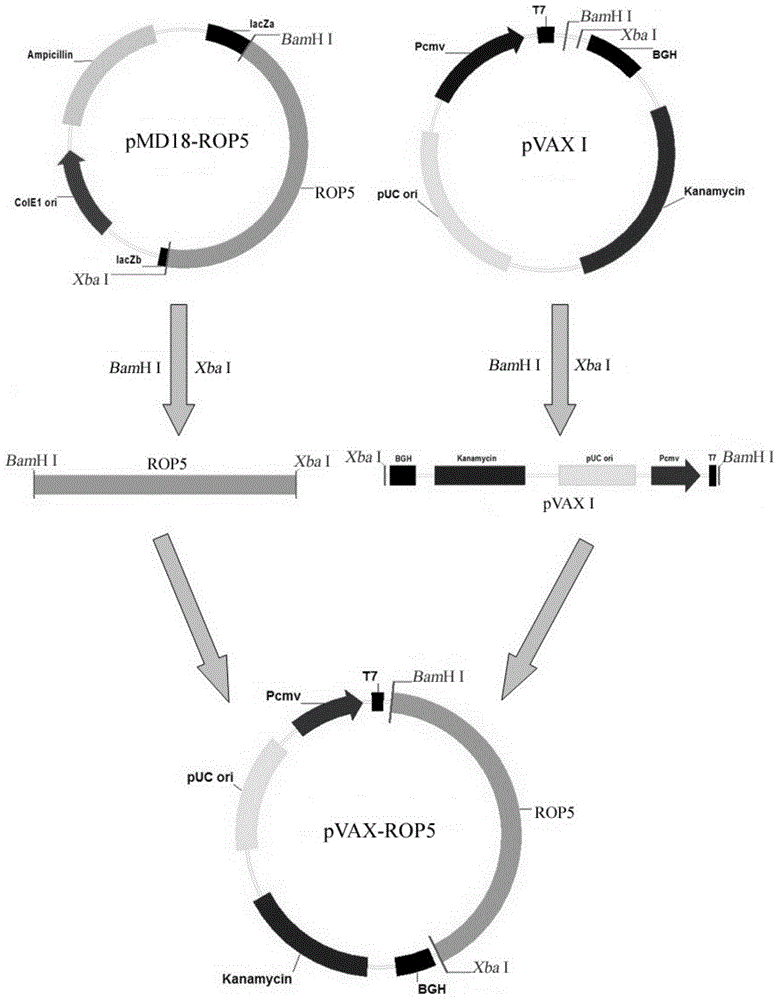
Vaccine for preventing toxoplasma infection and application thereof
ActiveCN103330947AInfection Prevention and ControlImprove immunityProtozoa antigen ingredientsGenetic material ingredientsAntigenImmune effects
The invention provides a vaccine for preventing toxoplasma infection. The vaccine contains nucleotide sequences selected from the followings: 1) 736-2385th nucleotide sequences as shown in SEQ ID No.1, and / or 759-2411th nucleotide sequences as shown in SEQ ID No.3; 2) nucleotide sequences complementary with the nucleotide sequences as shown in 1); and 3) nucleotide sequences which perform substituting, deletion and addition modification on one or a plurality of basic groups of the nucleotide sequences as shown in 1) or 2). The vaccine has the capability of effectively preventing and controlling the infection of a toxoplasma animal model (Kunming mice), and the combination of recombinant plasmids can effectively improve the immune effect of a single-gene vaccine, therefore, plasmids pVAX-ROP5 and pVAX-GRA15 can be used as DNA (Deoxyribose Nucleic Acid) vaccine candidate antigens for preventing the toxoplasma infection; and a combined immune vaccine of a recombinant plasmid based on such two genes can be used as a cocktail composite vaccine for preventing the toxoplasma infection.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
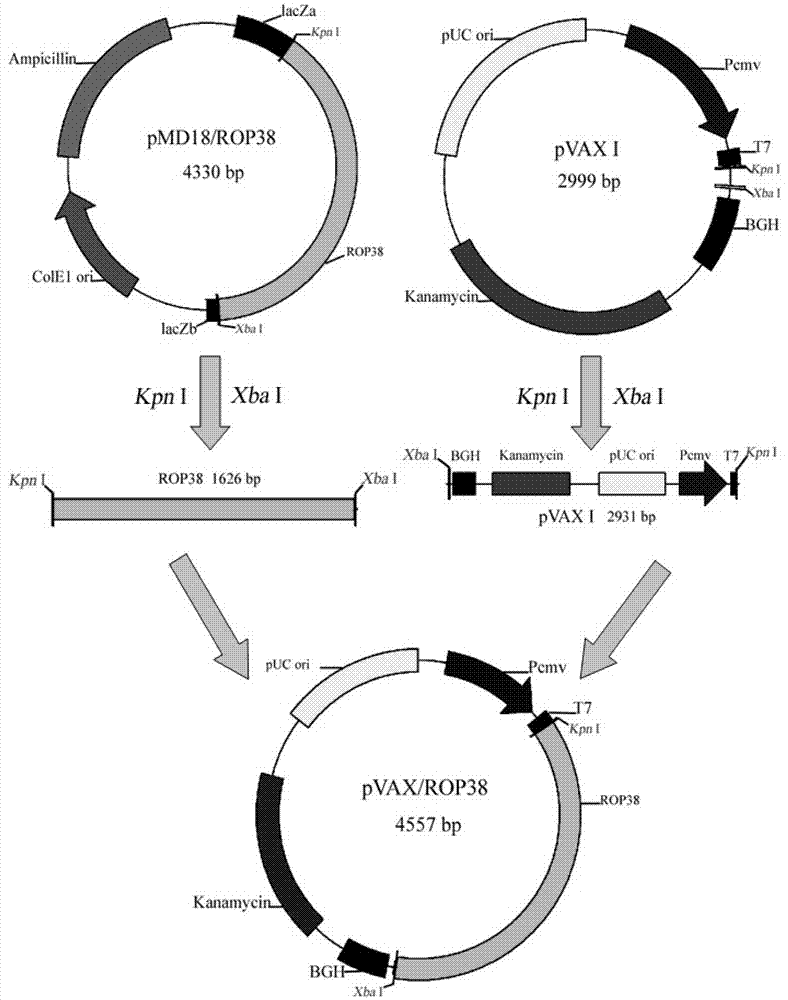
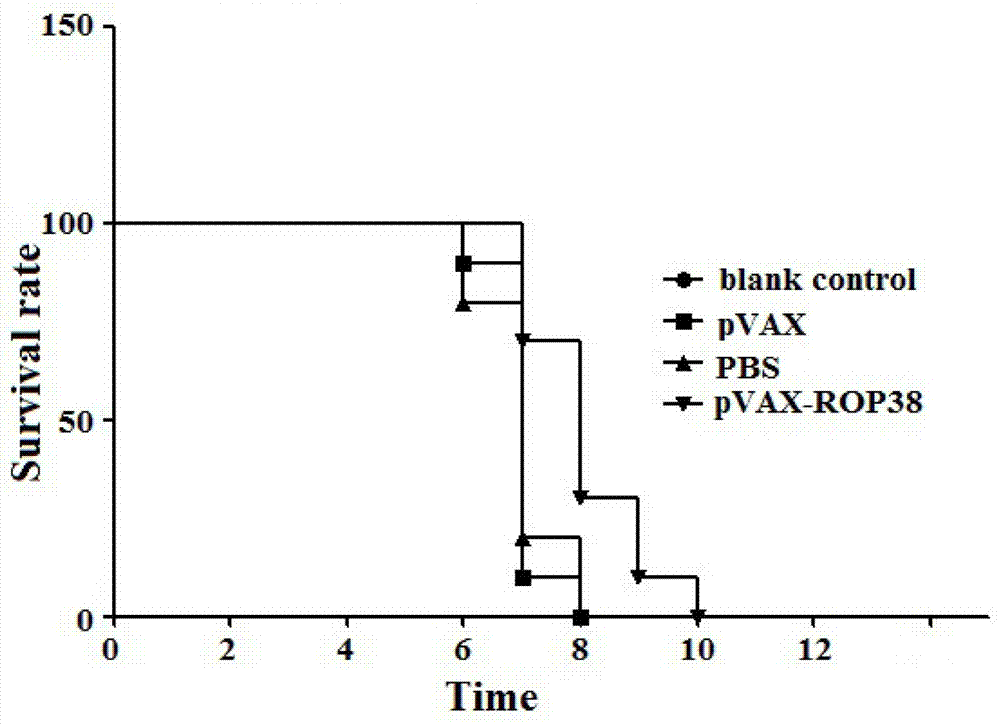
Vaccine for prevention of toxoplasma infection as well as preparation method and application of vaccine
InactiveCN103937818AInfection Prevention and ControlProlong survival timeProtozoa antigen ingredientsGenetic material ingredientsAntigenNucleotide
The invention provides a nucleotide sequence for prevention of toxoplasma infection. The nucleotide sequence for prevention of toxoplasma infection contains nucleotide sequences selected from (1) a nucleotide sequence as shown in SEQ ID No.1 of a sequence table, (2) a nucleotide sequence complementary with the nucleotide sequence as shown in the (1), and (3) a nucleotide sequence obtained by replacing, deleting, adding and modifying one or multiple of basic groups of the nucleotide sequence as shown in the (1) or (2) and having a function of preventing toxoplasma infection. The invention also provides a vaccine which comprises the nucleotide sequence and a medically acceptable carrier. The plasmid DNA vaccine pVAX-ROP38 can be used for effectively preventing and controlling infection of toxoplasma animal models (Kunming strain mouse), namely can be used for effectively prolonging the survival time of the mouse and reducing the worm burden of brain capsules. Therefore, the plasmid pVAX-ROP38 can serve as a DNA vaccine candidate antigen for resisting toxoplasma infection.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Surface antigen 1 of Toxoplasma gondii human antibody Fab fragment and encoded gene thereof
The present invention belongs to the field of biotechnology, and relates to a surface antigen 1 (SAG1) of Toxoplasma gondii human antibody Fab fragment, encoded gene and use thereof. According to the invention, the surface antigen 1 (SAG1) of Toxoplasma gondii human antibody Fab fragment is filtered from a base through establishing a Toxoplasma gondii human immunoglobulin, ELISA, diluting the prothrombin time, sequencing analysis, etc. Through expression purifying and authenticating, the human antigen Fab fragment is authenticated to specifically identify the tachyzoite-bradyzoite recombination SAG1 of Toxoplasma gondii and have higher affinity with the tachyzoite-bradyzoite recombination SAG1 of Toxoplasma gondii, for being identified with the specificity of Toxoplasma gondii tachyzoite-bradyzoite. The human antigen Fab fragment of the invention does not contain Fc segment and does not activate the alexin or cause the histopathological damages of human immune response, etc. when the function of restricting the invasion of Toxoplasma gondii to the host cell is exerted. The surface antigen 1 (SAG1) of Toxoplasma gondii human antibody Fab fragment is safe and reliable when applied for the human body. The antigen medicine for treating toxoplasmosis or the antigen targeted medicine can be prepared.
Owner:FUDAN UNIV
Inhibitors of Dihydrofolate Reductase With Antibacterial Antiprotozoal, Antifungal and Anticancer Properties
The compositions and methods described herein discloses the design, synthesis and testing of compounds that act as inhibitors of DHFR. The basic scaffold of these inhibitors includes a 2,4-diaminopyrimidine ring with a propargyl linker to another substituted aryl, bicyclo or heteroaryl ring. These DHFR inhibitors are potent and selective for many different pathogenic organisms, including the DHFR enzyme from bacteria such as Bacillus anthracis and methicillin-resistant Staphylococcus aureus, fungi such as Candida glabrata, Candida albicans and Cryptococcus neoformans and protozoa such as Cryptosporidium hominis and Toxoplasma gondii. These compounds and other similar compounds are also potent against the mammalian enzyme and may be useful as anti-cancer therapeutics.
Owner:UNIV OF CONNECTICUT
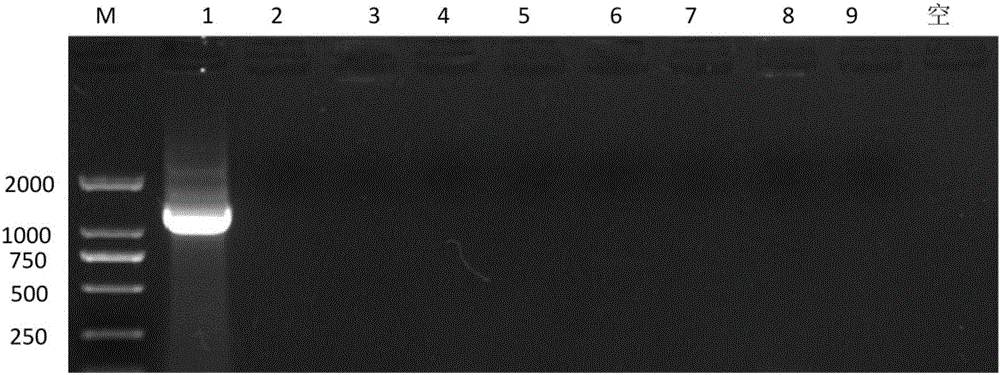
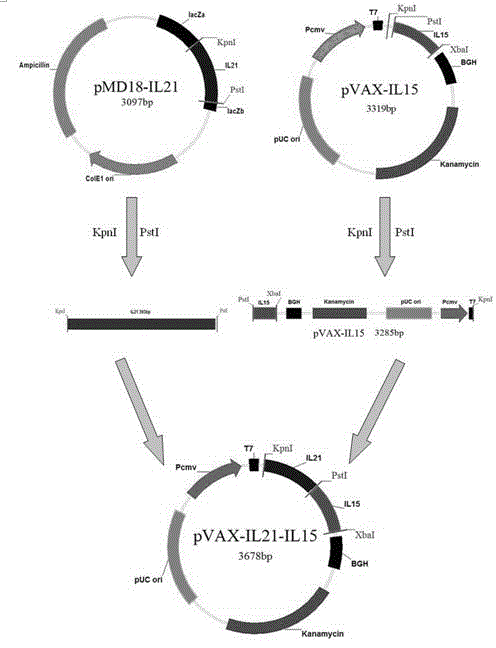
Immunologic adjuvant for preventing toxoplasma infection and application of immunologic adjuvant
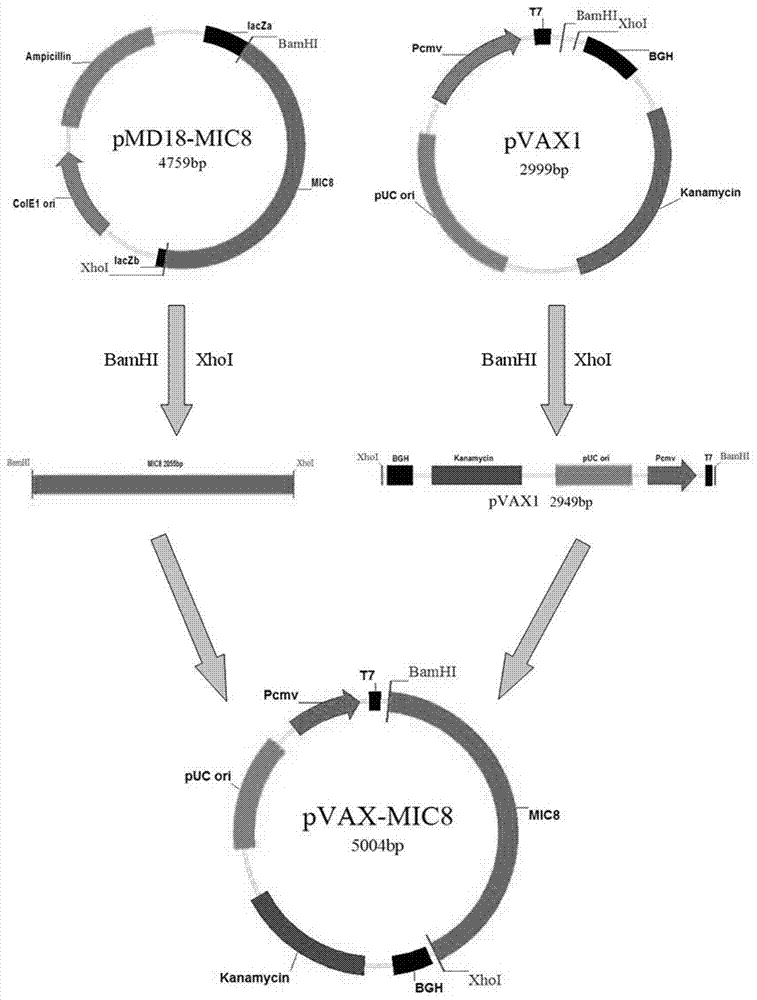
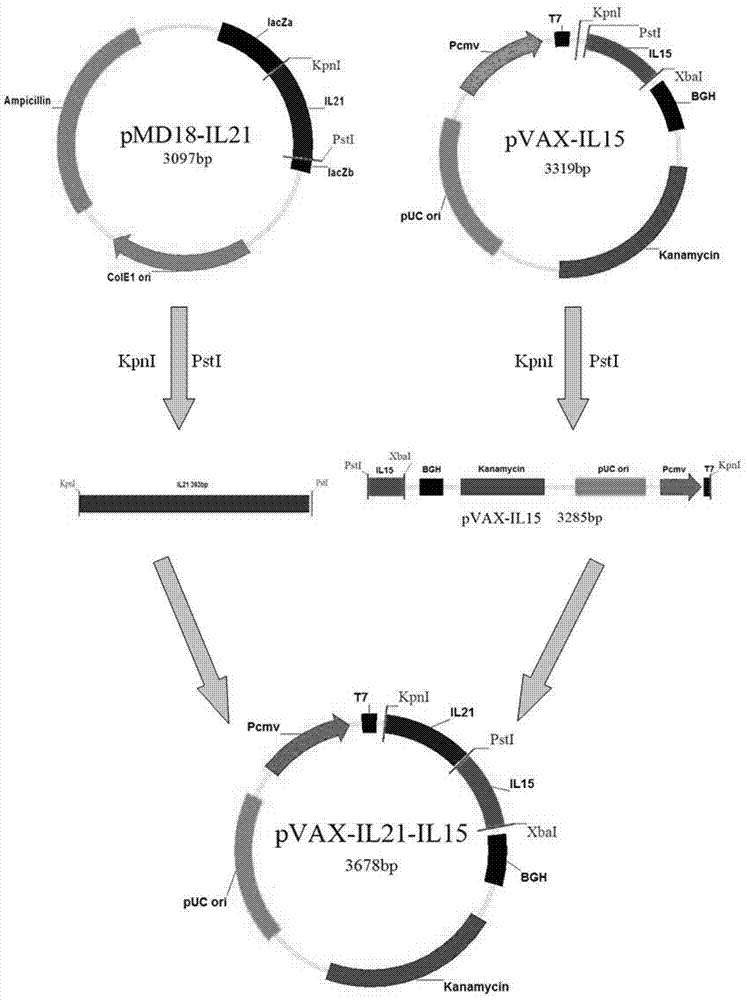
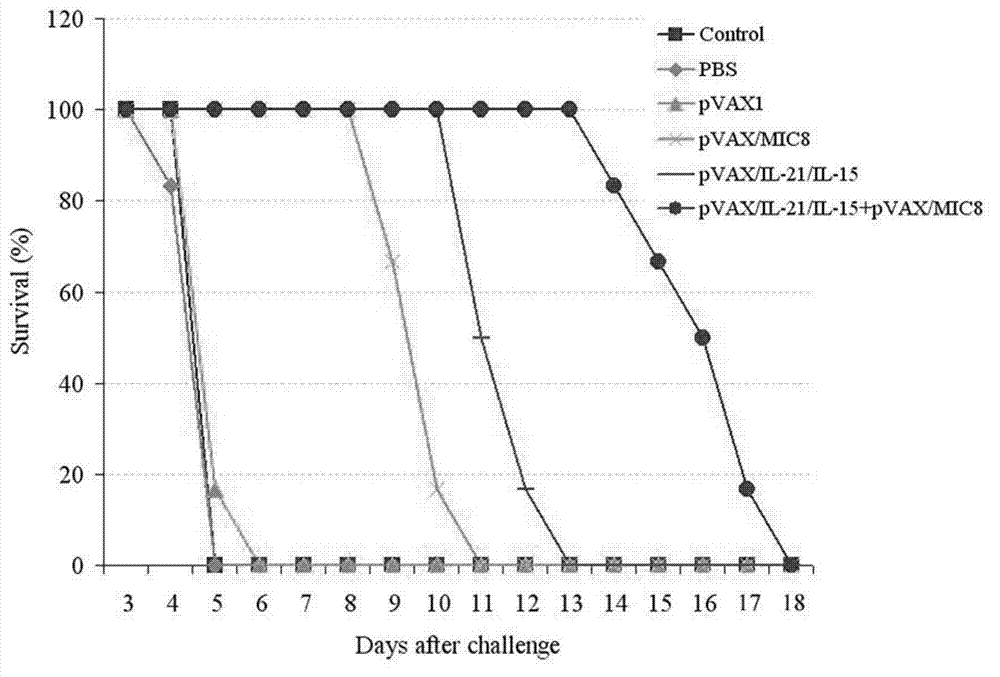
The invention discloses an immunologic adjuvant for preventing toxoplasma infection. The immunologic adjuvant is characterized in that the immunologic adjuvant is recombinant plasmids pVAX-IL21-IL15 formed by fusing interleukins IL21 and IL15 by using pVAX1 plasmids as carriers, and the base sequence of the immunologic adjuvant is represented by SEQ ID NO:1. The immunologic adjuvant for preventing the toxoplasma infection has a function of obviously improving the immune effect of MIC8 genes, so that the immune effect of an immune prevention vaccine against toxoplasma can be obviously enhanced.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Vaccine for preventing toxoplasma infection as well as preparation method and application thereof
InactiveCN104152466AInfection Prevention and ControlProlong survival timeProtozoa antigen ingredientsGenetic material ingredientsAntigenNucleotide
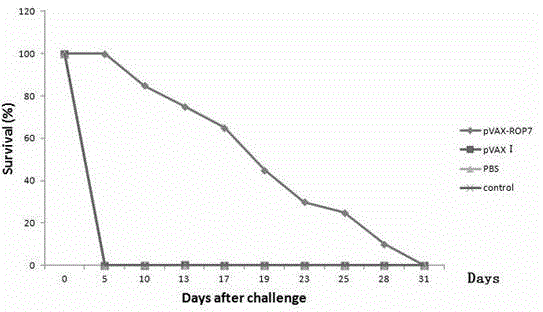
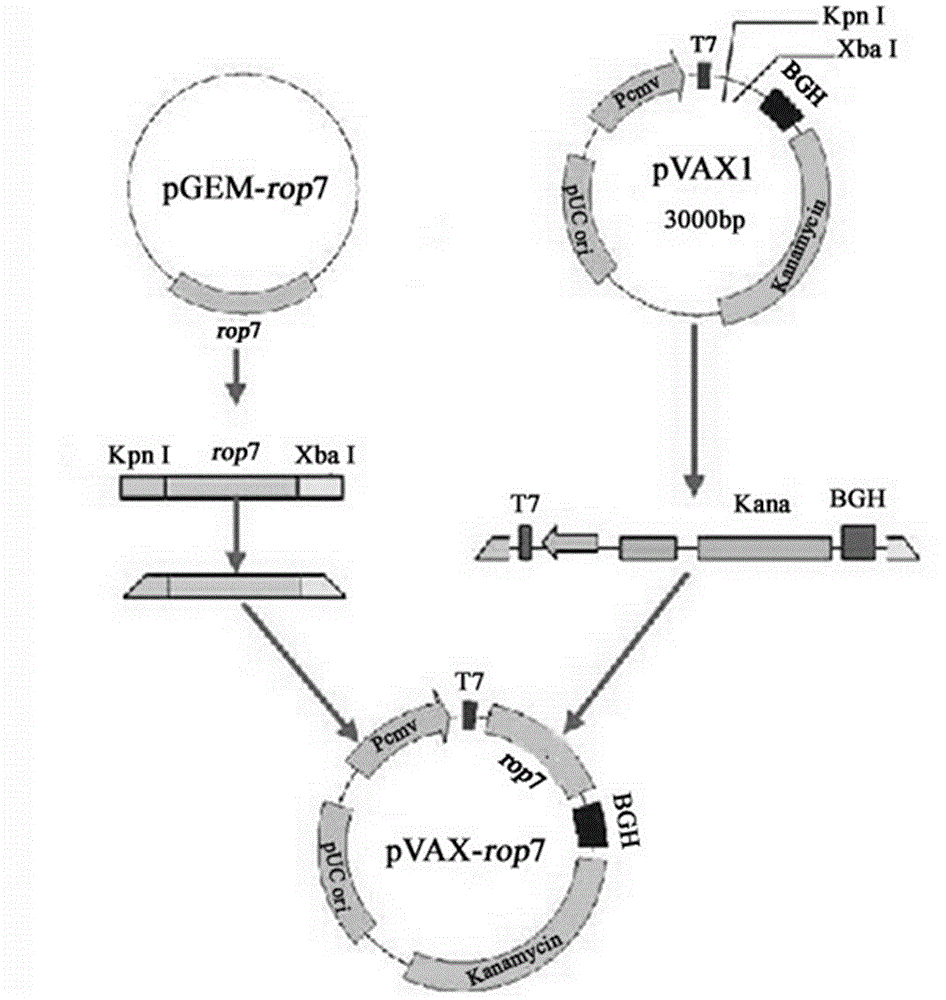
The invention firstly provides a nucleotide sequence for preventing toxoplasma infection. The nucleotide sequence is selected from one of the following nucleotide sequences: (1) a nucleotide sequence as shown in SEQ ID No.1 in a sequence table, (2) a nucleotide sequence which is complementary with the nucleotide sequence in (1) and (3) a nucleotide sequence which is obtained by substituting, deleting and modifying one or more basic groups of the nucleotide sequence as shown in (1) or (2) and has a function of preventing toxoplasma infection. The invention also provides a vaccine comprising the nucleotide sequence and a medically acceptable carrier. The plasmid DNA vaccine pVAX-ROP7 provided by the invention can be used for effectively preventing and controlling toxoplasma animal model (Kunming mouse) infection, namely effectively prolonging the survival time of a rat and reducing the polypide quantity of a brain cyst. Therefore, the plasmid pVAX-ROP7 can be used as a candidate antigen of DNA vaccine to prevent toxoplasma infection.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
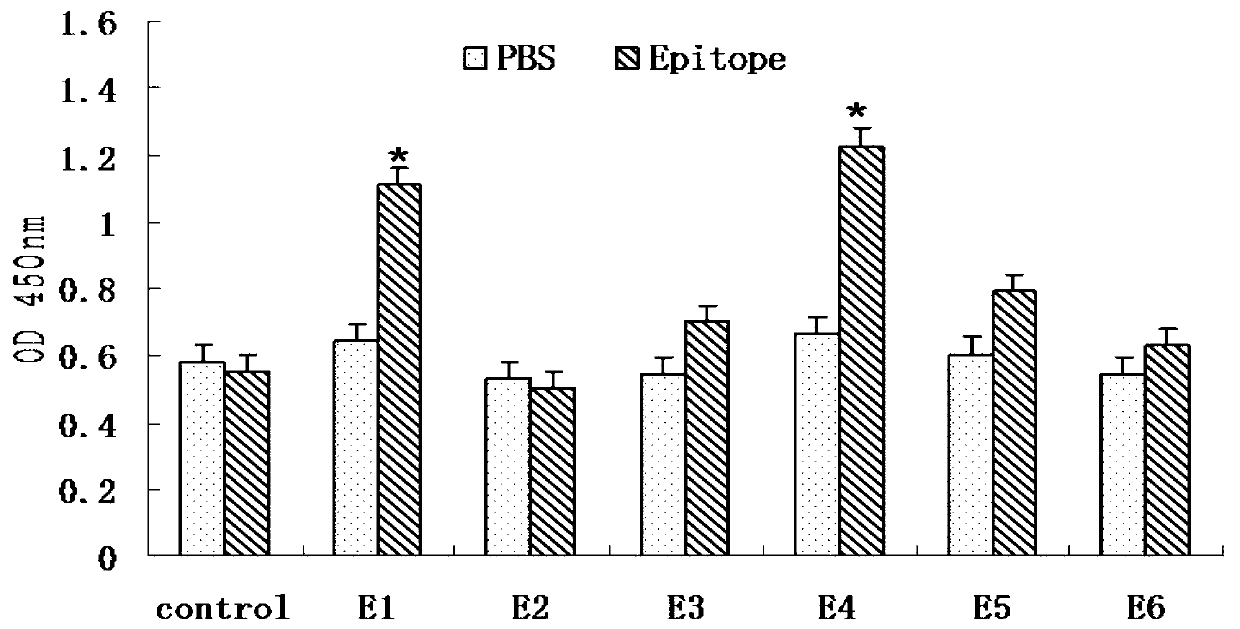
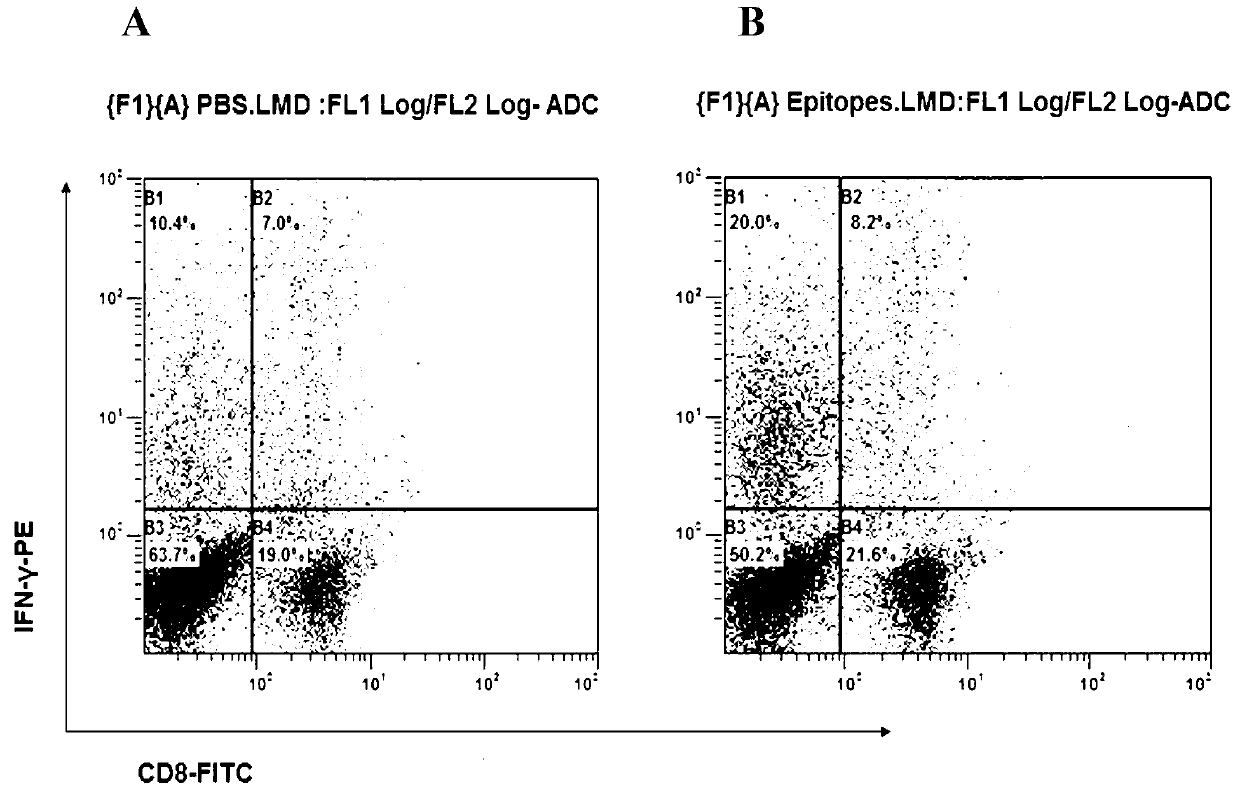
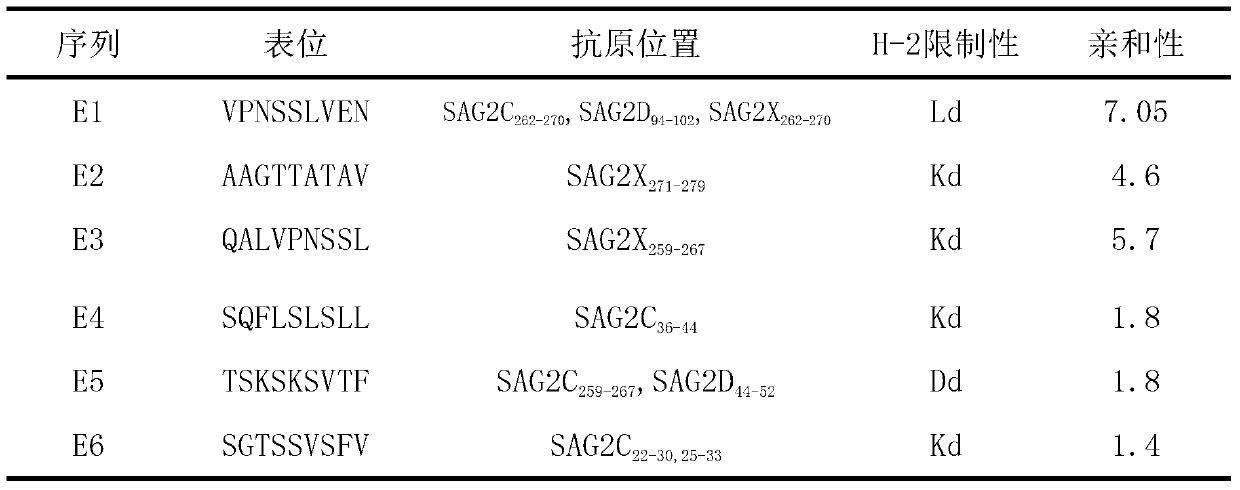
CD8+T cell dominant epitopes based on toxoplasmagondii bradyzoite antigens
ActiveCN103275182AProtozoa antigen ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsBALB/cActive immunization
The invention discloses two CD8+T cell dominant epitopes of VPNSSLVEN and SQFLSLSLL based on toxoplasmagondii bradyzoite specific surface antigens, wherein six CD8+T cell epitopes with H-2 restriction and high affinity are screened according to the protein sequences of the bradyzoite specific surface antigens of SAG2C, -2C and -2X; BALB / c mice are actively immunized by the screened epitope polypeptides; determinations for a lymphopoiesis level and a cell factor content, and an immune protection evaluation for epitope vaccines are performed after the immunization. The result indicates that powerful cell immunization can be generated by inducing the mice via the epitope vaccinesm, wherein the two antigen peptides of VPNSSLVEN and SQFLSLSLL are provided to be great in the capacity of stimulating T cell proliferation, as well as capable of inducing the mice to secrete protective cell factors and immune protection, being used as the epitope vaccines effectively resisting toxoplasmagondii infection, and reducing an encystations rate in the brain tissue of a host.
Owner:SHANDONG UNIV
Specific primers, probes, kit and chip for gene detection of Toxoplasma gondii
PendingCN110982880AConvenient diagnosis and treatmentEasy to monitorMicrobiological testing/measurementDNA/RNA fragmentationGondii toxoplasmaVirology
The invention relates to the field of molecular biology, and provides specific primers for detecting Toxoplasma gondii. The sequence of an upstream primer in the specific primers is as shown in SEQ IDNo. 1; the sequence of a downstream primer in the specific primers is as shown in SEQ ID No. 2. The invention also provides probes for detecting Toxoplasma gondii. The sequences of the probes are asshown in SEQ ID No. 3-6. The invention further provides a kit for detecting Toxoplasma gondii. The kit comprises the primers and the probes for detecting Toxoplasma gondii. The invention further provides a chip for detecting Toxoplasma gondii. The chip comprises the probes for detecting Toxoplasma gondii. According to the invention, gene chip detection is carried out on Toxoplasma gondii by usingthe primers and the probes, and differential diagnosis can be specifically carried out on Toxoplasma gondii infection conditions.
Owner:中国疾病预防控制中心寄生虫病预防控制所国家热带病研究中心
Primer, probe and kit for detecting pneumocystis carinii and toxoplasma gondii and detection method thereof
ActiveCN111926007APrevention and Control of TransmissionHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationGondii toxoplasmaPharmaceutical drug
The invention discloses a primer, a probe, a kit and a detection method for detecting pneumocystis carinii and toxoplasma gondii, and particularly relates to a kit and a detection method for double fluorescent quantitative PCR detection of pneumocystis carinii and toxoplasma gondii. According to the invention, the primer pair and the probe are designed by utilizing conserved regions of pneumocystis carinii and toxoplasma gondii, the primer pair and the probe which can specifically amplify pneumocystis carinii and toxoplasma gondii are screened out, the sensitivity is high, the two pairs of primers and the two probes do not interfere with each other, and a sample only containing 1 copy can be accurately detected; by utilizing the primer pair, the probe and the detection method, the contentsof the pneumocystis carinii and the toxoplasma gondii can be absolutely quantified; the kit is simple in structure, safe in detection, rapid and convenient, can be used for detecting the pneumocystiscarinii and the toxoplasma gondii and tracking and monitoring the treatment effect of medicines of a patient, and is beneficial to carrying out epidemiological investigation and preventing and controlling propagation of the pneumocystis carinii and the toxoplasma gondii.
Owner:JIANGSU BIOPERFECTUS TECH CO LTD +1
Kit for rapid detection of eperythrozoon, leptospira and toxoplasma in blood and application of kit
InactiveCN103789430AThe detection process is fastEasy to operateMicrobiological testing/measurementAgainst vector-borne diseasesFalse positive ratePathogen
The invention discloses a kit for rapid detection of eperythrozoon, leptospira and toxoplasma in blood and application of the kit. The kit consists of a detachable 96-pore polymerase chain reaction (PCR) tube, PCR reaction liquid, TaqDNA polymerase, standard and control, wherein the PCR reaction liquid contains a PCR buffer solution, dNTPs, MgCl2, KCl, gelatin and various detection primers; the various detection primers are respectively specific primers for eperythrozoon, leptospira and toxoplasma. The kit is applicable to detecting whether blood is infected by one or more pathogens in the eperythrozoon, leptospira and toxoplasma. The kit disclosed by the invention is rapid in detection speed, high in detection sensitivity, good in specificity, and simple and convenient to operate; and the kit can simultaneously identify and detect one or more specimen in mixed infection of the eperythrozoon, leptospira and toxoplasma, thus greatly improving detection accuracy and reduce false positive rate.
Owner:ZHEJIANG UNIV
Kit for absolutely quantitatively detecting Toxoplasma gondii based on digital PCR and detection method thereof
InactiveCN106434992AEfficient detectionEasy to determineMicrobiological testing/measurementMicroorganism based processesTest sampleMicrobiology
The invention relates to a kit for absolutely quantitatively detecting Toxoplasma gondii based on digital PCR and a detection method thereof; the kit is composed of a microdrop digital PCR kit and a plurality of microdrop generator sheets; the detection method is characterized by comprising: (1) extracting total DNA of a test sample; (2) using the total DNA as a template to carry out PCR amplification; (3) placing the PCR production and microdrop reader to read signals, analyzing experimental data to obtain an absolute content of Toxoplasma gondii in the sample. The kit and method can provide direct quantitation in Toxoplasma gondii detection, have no need for standard curves, have the advantages, such as good operational convenience, high speed and efficiency, good specific sensitivity and low cost, etiological diagnosis on Toxoplasma gondii can be effectively carried out, detection rate is increased, the common problems of existing Toxoplasma gondii detection kits, such as low sensitivity and high misdiagnosis rate, are solved, and the kit and method are suitable for clinical investigations and large-scale disease detection and supervision, related preventive and control measures can be collected in time, and economic loss is decreased.
Owner:吉林省畜牧兽医科学研究院
Nucleotide sequence for preventing infection of toxoplasmas and application of nucleotide sequence
InactiveCN104830850APromote aggregationInhibitionOrganic active ingredientsPeptide/protein ingredientsImmune effectsNucleotide
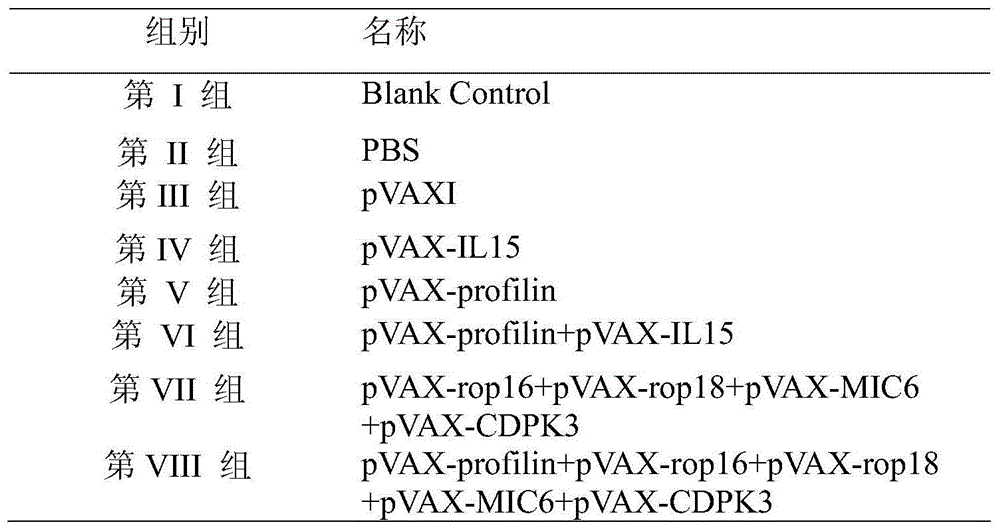
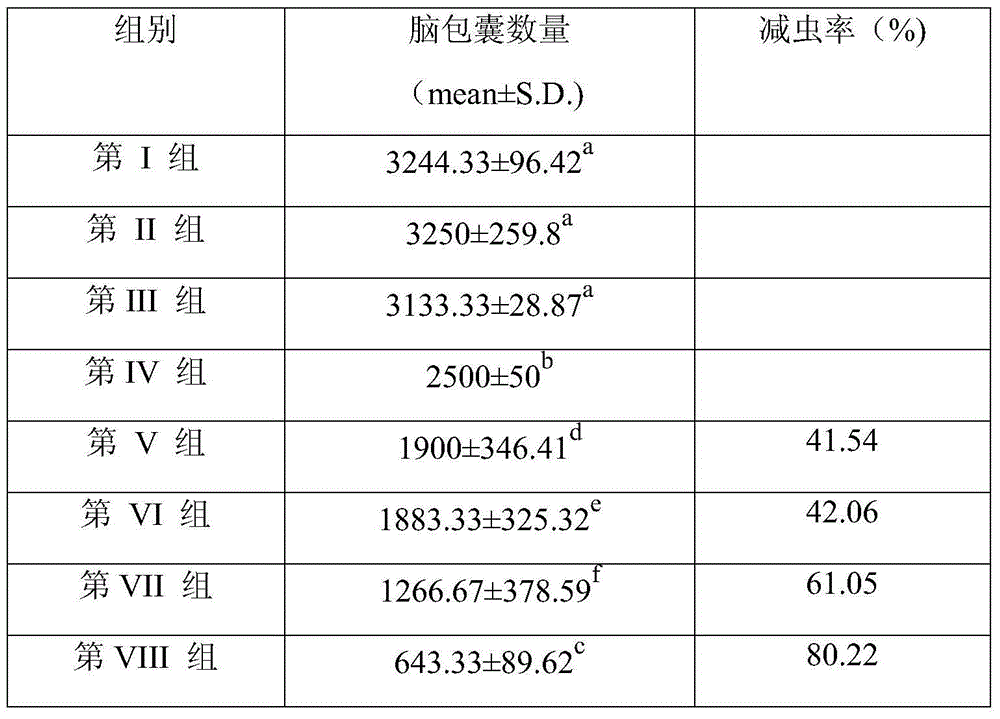
The invention provides a nucleotide sequence for preventing infection of toxoplasmas. The nucleotide sequence contains any one group of the following nucleotide sequences: (1) a nucleotide sequence of profilin; (2) nucleotide sequences of profilin, rop16, rop18, MIC6 and CDPK3; (3) nucleotide sequences of profilin and IL15; (4) nucleotide sequences of rop16, rop18, MIC6 and CDPK3; (5) complementary sequences of the nucleotide sequences from (1) to (4); and (6) nucleotide sequences prepared through carrying out substitution, deletion, addition and modification on nucleotide sequences from (1) to (5). By virtue of a pVAX-profilin vaccine and a combined vaccine, the generation of brain cysts of the toxoplasmas can be effectively inhibited, and a relatively good immune effect is achieved.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Toxoplasma indirect hemagg lutination diagnostic reagent and producing process thereof
The insect indirect blood diagnosis regent and its making method are made by the antigen secreted by the bow form insect. It inoculates the 1*10-5-1*10-6 bow form insect into the small rat belly cavity, then to soak in the alcohol to disinfect the body surface after making the small white rat death to collect a belly cavity liquid by the centrifugation; the red cell treated by the secreted antigen which is the bow insect indirect blood diagnosis antigen; the 5% red cell treated by the secreted antigen are suspended in the water bath box, then to centrifugate and wash for 3-5 times; then to wash by the 2% NRS of 0.15M PBS with the pH7.2 for 2-3 times. the sediments is to matched to the 1% sensitized red cell suspension with the 0.15M PBS of pH7.2 containing 2% NRS. It is dried by the freeze drier and vacuumize to get the indirect blood diagnosis regent with the IHA secreted by the bow form insect. The invention can be used for examining the bow form insect disease and the census aspect of bow form insect disease.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Semi-nested PCR method testing toxoplasma gondii
InactiveCN101245367AQuick checkAccurate detectionMicrobiological testing/measurementEpidemiologyImpurity
The invention provides a semi-nested PCR method for detecting toxoplasma gondii, which determines whether a sample is toxoplasma gondii infection positive or negative by arranging a PCR detection kit, extracting the DNA of a test sample and carrying out the semi-nested PCR amplification and the analysis of the amplified products. The method of the invention can rapidly and accurately detect the toxoplasma gondii in the test sample, and can also be used for the molecular epidemiological investigation and the efficacy monitoring of toxoplasmosis. The template DNA preparation steps of the method are simple, the cost is low, however, the conventional method needs to be processed by lysozyme, protease K, sodium dodecyl sulfate, cetyltrimethyl ammonium bromide and other reagents, the time is long and the cost is high. The method is established on the molecular biology, which can exclude the interferences of bacteria and impurity particles, thus greatly improving diagnostic accuracy and reducing false positive rate.
Owner:ZHEJIANG UNIV
Toxoplasma metabolic secretion antigen vaccine and its prepn process
ActiveCN1931363APositive rate of proliferative response increasedNo danger of spreading poisonBacterial antigen ingredientsAntiparasitic agentsAdjuvantCulture fluid
The present invention is toxoplasma metabolic secretion antigen vaccine and its preparation process. The toxoplasma metabolic secretion antigen vaccine is prepared with toxoplasma metabolic secretion antigen. The preparation process includes the following steps: inoculating toxoplasma tachyzoite to abdominal cavity of mouse, killing the mouse and soaking in alcohol for surface sterilizing; collecting polypide; obtaining abdominal cavity liquid via centrifuging and collecting supernatant; inoculating toxoplasma tachyzoite to vero cell to culture and collecting the cultured liquid after cell falling off via centrifuging and collecting supernatant; thrice freezing and thawing abdominal cavity liquid and cultured liquid; mixing, measuring protein content of the mixed liquid; mixing the mixed liquid with M206 adjuvant, emulsifying and packing as the composite toxoplasma vaccine with antigen protein content of 10 mg / ml. The vaccine is for inducing humoral immunity and cellular immunity.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Oral liquid for inhibiting and eliminating toxoplasma gondii and toxoplasma gondii cysts and preparation method of oral liquid
InactiveCN109908265AInhibition of reproductive activityInhibition of reproductionPharmaceutical delivery mechanismAntiparasitic agentsBiotechnologySmoked Plum
The invention discloses oral liquid for inhibiting and eliminating toxoplasma gondii and toxoplasma gondii cysts and a preparation method of the oral liquid. The oral liquid comprises, by weight, 2-4parts of Chinese wolfberries, 12-16 parts of smoked plums, 15-20 parts of the root of Indian pokeweed, 50-60 parts of herba artemisiae annuae, 10-15 parts of dandelions, 4-7 parts of rhizoma polygonati, 4-6 parts of radix bupleuri, 8-10 parts of white poria, 2-4 parts of cyrtomium fortunei, 3-5 parts of bletilla striata, 3-5 parts of branchlets and leaves of antifebrile di-chroa, 4-6 parts of fructus ulmi, 6-10 parts of frankincense, 6-8 parts of the root bark of white mulberry, 1-3 parts of white atractylodes rhizomes and 300-500 parts of purified water. The preparation method includes the following steps: extracting active ingredients of the raw materials and uniformly mixing the active ingredients with the purified water. The oral liquid can inhibit the breeding and activity of the toxoplasma gondii in human cells after being continuously taken, thereby preventing the infection of the toxoplasma gondii, treating toxoplasmosis, and expelling the cysts and pseudocysts of the toxoplasma gondii.
Owner:ZHUHAI HANTANG BIOTECH ENG CO LTD
Method for detecting PCR of toxoplasmas
InactiveCN101649348ASimplify and standardize diagnostic methodsAvoid pollutionMicrobiological testing/measurementElectrophoresisBuffer solution
The invention discloses a method for detecting PCR of toxoplasmas. The detecting method comprises the following steps: (1) mixing a primer, Taq DNA polyase, a polyase stabilizer, four mononucleotides,a buffer solution and a sampling buffer solution before a PCR product is subjected to electrophoresis, and lyophilizing the mixture into powder; (2) mixing the powder obtained in the step (1) and a sample to perform a PCR reaction so as to obtain the PCR product; and (3) performing electrophoresis detection on the PCR product. The sample comprises nucleic acid which is extracted from mammalian urine and / or faeces.
Owner:SHANGHAI RES CENT FOR MODEL ORGANISMS
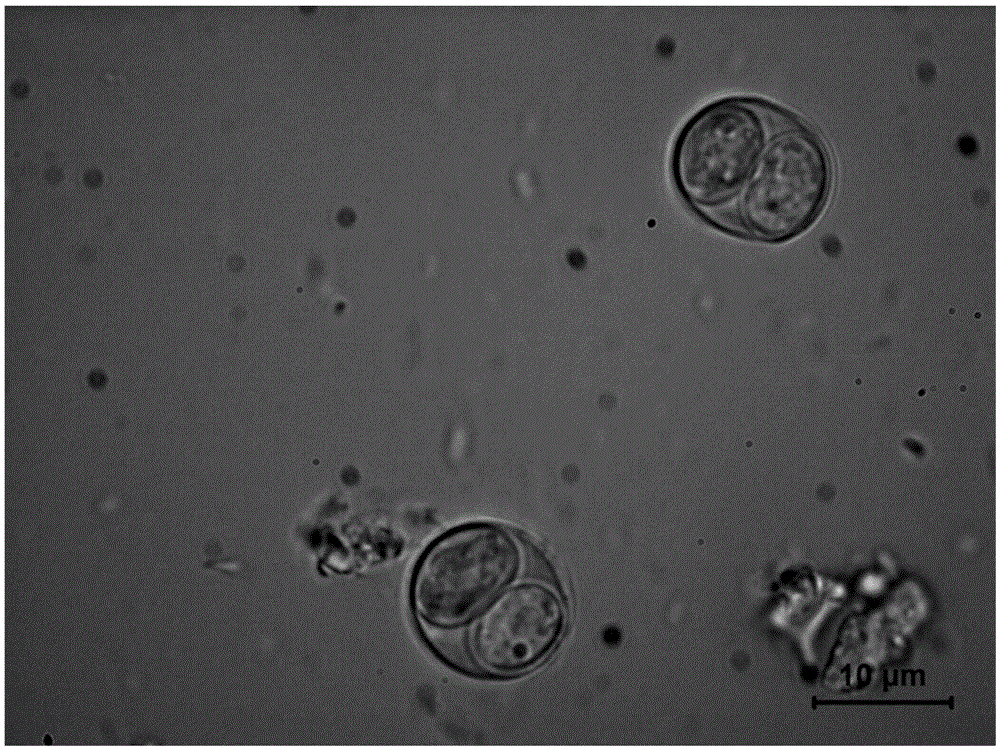
Preparation, purification and storage method of toxoplasma gondii oocysts
The invention discloses a preparation, purification and storage method of toxoplasma gondii oocysts. The method comprises the following steps of A, acquiring a toxoplasma gondii cyst; B, acquiring a toxoplasma gondii negative cat; C, feeding the cat with the toxoplasma gondii cyst; D, collecting excrement from 1 to 21 days; E, separating the toxoplasma gondii oocysts from the excrement; F, storing the toxoplasma gondii oocysts. The toxoplasma gondii oocysts disclosed by the invention are good in activity, can be stored in a fridge of 4 degrees for a long time, and a storage period is longer than 5 years, a good research and preparation material for a toxoplasma gondii vaccine can be provided, a range is relatively small, and contacted materials and utensils are subjected to harmless treatment, so that environment pollution is avoided.
Owner:HENAN AGRICULTURAL UNIVERSITY
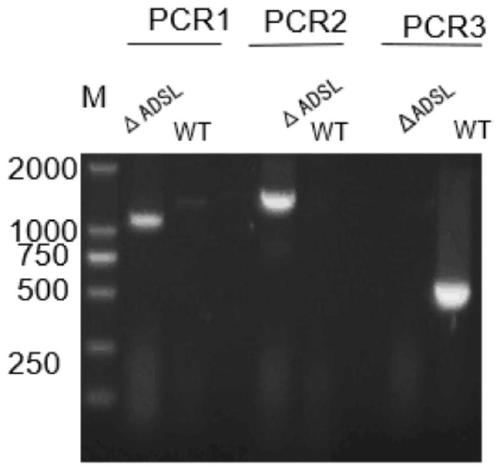
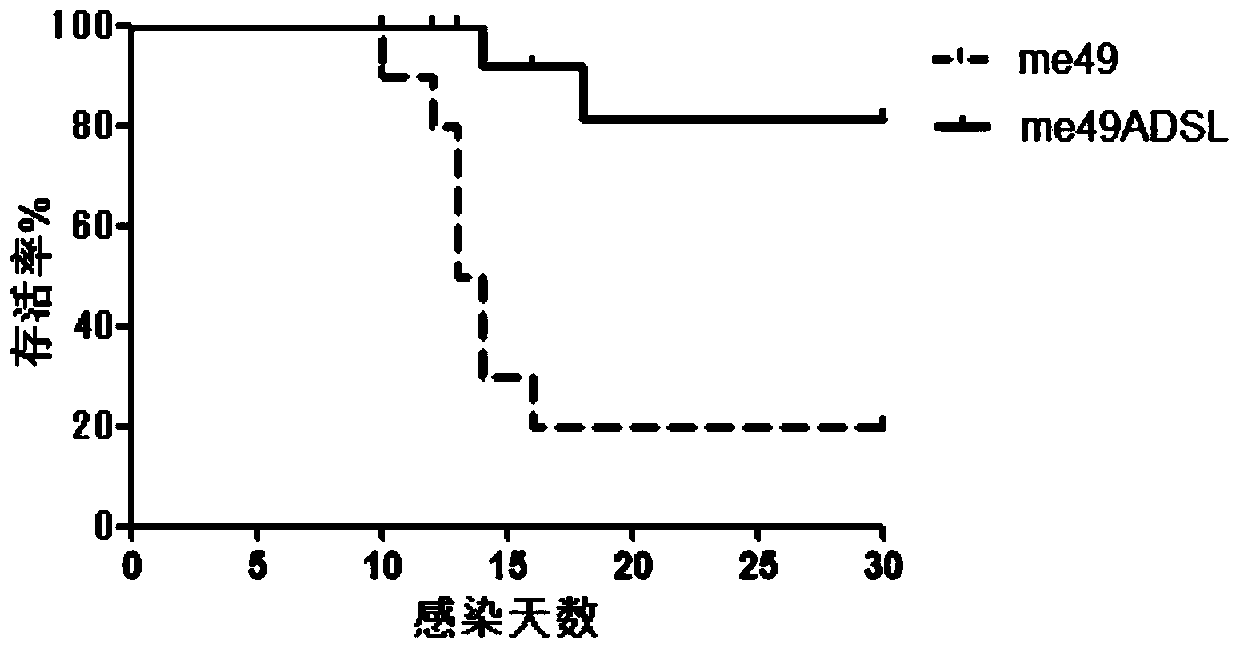
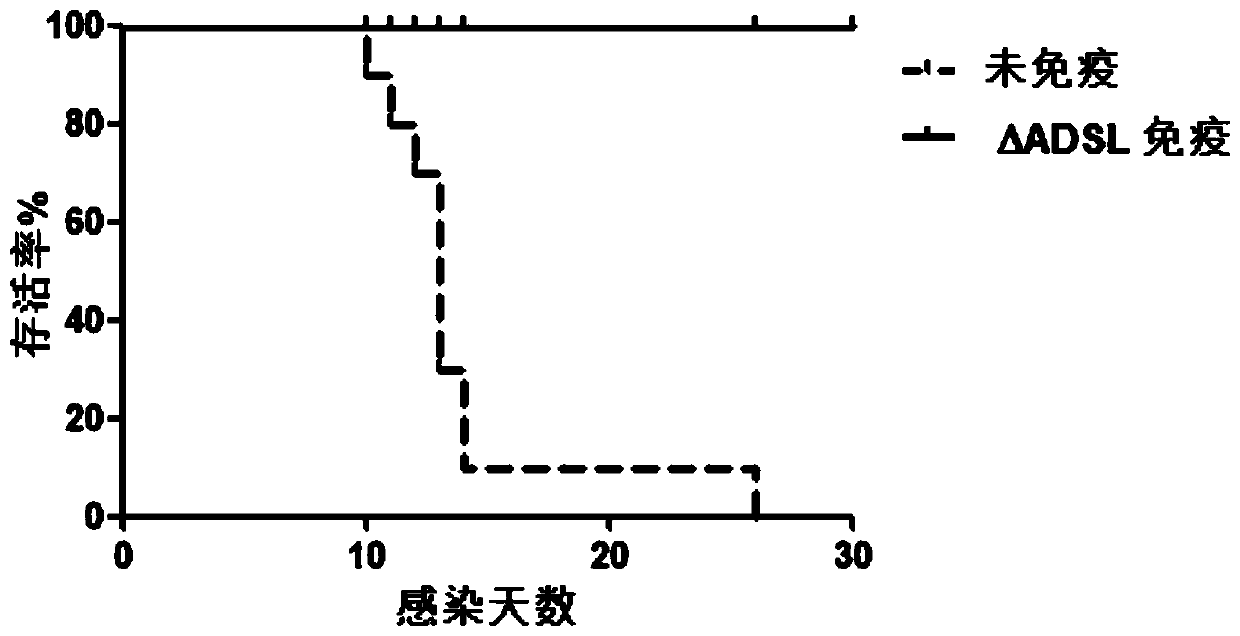
Construction method and applications for toxoplasma adenylosuccinate lyase gene knockout strains
ActiveCN110093277AImprove immunityLow toxicityCarbon-nitrogen lyasesProtozoaEnzyme GeneVirulent characteristics
The invention discloses a construction method for toxoplasma gene knockout strains. The method knocks out a toxoplasma adenylosuccinate enzyme gene; the nucleotide sequence of the adenylosuccinate enzyme gene is shown as SEQ ID NO : 1; and a toxoplasma gene knockout strain has a nucleotide sequence shown as SEQ ID NO : 2 at an adenylosuccinate enzyme site. The gene knockout strain has the advantage of being small in virulence, can enhance the immunocompetence of animals to toxoplasma, and has the potential to become an anti-toxoplasma genetic engineering vaccine.
Owner:枝江市宜合众畜牧有限公司
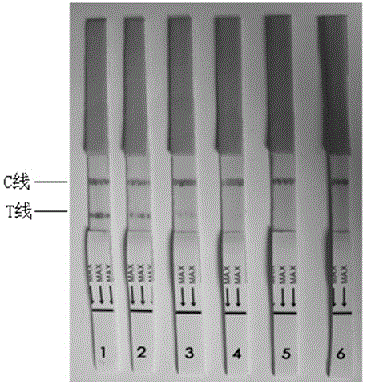
Toxoplasma detection kit
The invention provides a monoclonal hybridoma cell strain 4D5.The collection number is CCTCC NO: C201624, the classification name is hybridoma cell strain No.4D5, and the collection date is February 1st, 2016.The invention further provides a toxoplasma detection kit.The toxoplasma detection kit is formed by a test strip, a standard sample, a reference sample and a sample diluent.An anti-toxoplasma SAG3 monoclonal monomer embedded in the test strip is secreted by the monoclonal hybridoma cell strain 4D5 with the collection number CCTCC NO: C201624.According to the toxoplasma detection kit, the immunochromatographic strip technology is adopted, and the technology is short in detection time, low in cost, easy to operate and suitable for field detection and epidemiological surveying; toxoplasma SAG3 serves as a diagnostic antigen; compared with SAG1 and SAG2, the expression quantity of the SAG3 is large, the toxoplasma SAG3 can be expressed in all infection stages of toxoplasma and more suitable for serving as the diagnostic antigen; the clinical application result shows that the toxoplasma detection kit is high in sensibility and specificity, good in stability and easy to popularize to the primary level.
Owner:ZHEJIANG UNIV
Anti-toxoplasma composition drug and screening method thereof
ActiveCN104998277AGood against Toxoplasma gondiiClean up thoroughlyOrganic active ingredientsAntiparasitic agentsSulfamonomethoxineSulfanilamide
The invention discloses an anti-toxoplasma composition drug and a screening method thereof, and belongs to the field of medicine. According to the anti-toxoplasma composition drug and the screening method thereof, mouse test toxoplasmosis animal models are established, the ten drugs of a sulfadiazine sodium injection, a compound sulfamethoxazole, a lincomycin hydrochloride injection, a sulfamonomethoxine sodium injection, a florfenicol injection, acetylspiramycin, pyrimethamine, roxithromycin, artemisinin and radix sophorae flavescentis are selected out, two compositions which have the best anti-toxoplasma effect are screened out, one composition comprises the sulfamonomethoxine, the pyrimethamine and TMP, and the other composition comprises the compound sulfamethoxazole and the acetylspiramycin; animal models in mice and pigs are established, it is verified that the two compositions are both effective on the treatment of artificially infected toxplasmosis in pigs, and the treatment effect on the pigs is in accordance with that of the mouse models. By means of the anti-toxoplasma composition drug and the screening method thereof, it is proved that the the feasible method is achieved by utilizing the mouse test toxoplasmosis models to evaluate the efficacy of drugs on treating toxplasmosis in pigs, and meanwhile two anti-toxoplasma drug compositions with good effects are provided.
Owner:SOUTH CHINA AGRI UNIV
Specific detection primer for swine toxoplasmosis and detection kit
InactiveCN106834504ARealize epidemiological investigationStrong specificityMicrobiological testing/measurementMicroorganism based processesSpecific detectionToxoplasmosis
The invention relates to the technical field of preparation of disease detection reagents and discloses a specific detection primer for swine toxoplasmosis and a detection kit. A dense granular protein 14 (GRA14) gene is taken as a target gene for diagnosis of the swine toxoplasmosis, specific detection primer GRAF-3 and GRAR-3 for toxoplasmosis are designed and screened out, and the sequence of each of the GRAF-3 and GRAR-3 is as shown as in SEQ ID No:1 and SEQ ID No.2. The toxoplasmosis is detected through the primer which is highly specific and highly flexibly, the result of a PCR (polymerase chain reaction) is easy to judge, epidemiological investigation for the toxoplasmosis and detection and clinical diagnosis of invisible infection can be realized by the aid the detection kit for the toxoplasmosis, prepared from the primer, and a foundation is laid for further research on diagnostic and preventive techniques and the like of the toxoplasmosis.
Owner:SOUTH CHINA AGRI UNIV
Gene for preventing toxoplasma infection and application of gene
ActiveCN103333900AInfection Prevention and ControlImprove immunityHydrolasesGenetic material ingredientsAdjuvantCalcium-dependent protein kinase
The invention provides a nucleotide sequence for preventing toxoplasma infection. The nucleotide sequences selected from the followings are contained: 1) 724-1470th nucleotide sequences as shown in SEQ ID No.3, and / or 724-2472nd nucleotide sequences as shown in SEQ ID No.1; 2) nucleotide sequences complementary with the nucleotide sequences as shown in 1); and 3) nucleotide sequences which perform substituting, deletion and addition modification on one or a plurality of basic groups of the nucleotide sequences as shown in 1) or 2). The pVAX-CDPK (Calcium Dependent Protein Kinase)1 can effectively prevent and control the infection of a toxoplasma animal model (Kunming mice), and pVAX-IL21-IL15 not only can be independently used for effectively preventing and controlling the toxoplasma infection, but also can be used as a cytokines adjuvant for effectively improving the immune effect of a vaccine pVAX-CDPK1.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Heterocyclic analogs of propargyl-linked inhibitors of dihydrofolate reductase
ActiveUS8853228B2Increase capacityStraightforward to synthesizeAntibacterial agentsOrganic active ingredientsBacteroidesProtozoa
Owner:UNIV OF CONNECTICUT
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com