Specific detection primer for swine toxoplasmosis and detection kit
A technology for detection of toxoplasmosis and primers, applied in biochemical equipment and methods, measurement/testing of microorganisms, microorganisms, etc., can solve the problem of sensitivity, specificity and clinical application of unidentified pig toxoplasmosis infection Effect and other issues, to achieve high sensitivity, strong specificity, easy to determine the effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
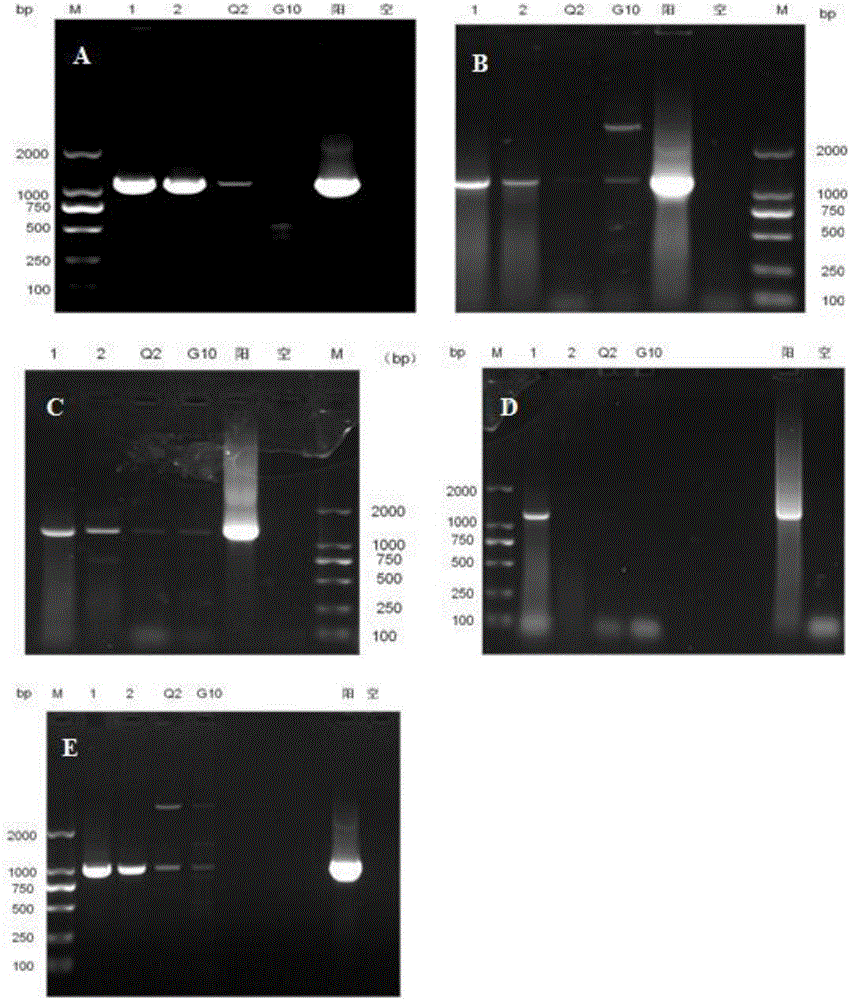
[0050] Example 1 Primer design and screening
[0051] The dense granule protein 14 (GRA14) gene was used as the target gene for the diagnosis of toxoplasmosis, and specific primers were designed according to the GRA14 gene sequence, as shown in Table 1.
[0052]
[0053] The PCR reaction system is: Premix E×Taq Version 2.0 (Loading dye Mix) 12.5 μL, forward primer and reverse primer 0.5 μL each, double distilled water 9.5 μL, template DNA 2 μL, number of amplified samples n (n=number of samples +3). Mix the above reaction solution in a centrifuge tube, mix well, and dispense. Take each sample DNA into the corresponding reaction tube, mark each reaction tube, mix and centrifuge, and place it on the PCR machine for reaction.
[0054] PCR amplification conditions are: (i) pre-denaturation at 94°C for 5 min; (ii) denaturation at 94°C for 30 s, annealing at 65°C for 30 s, a total of 35 cycles, (iii) extension at 72°C for 2 min, (iv) after 72°C Extend for 5min.
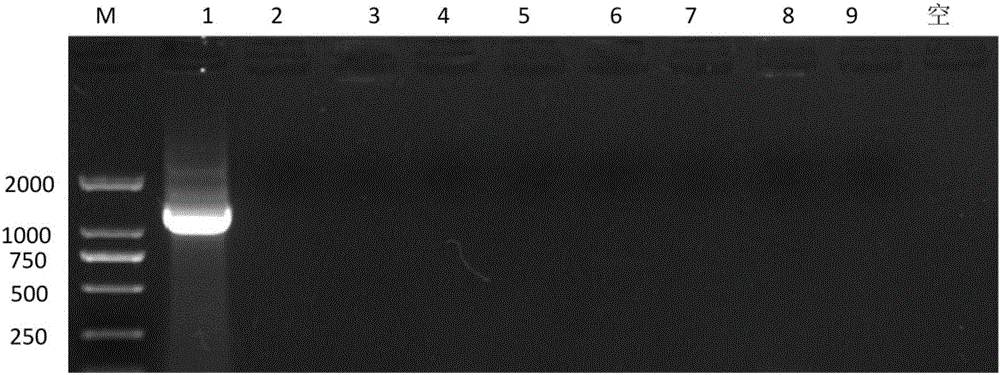
[0055] The results show ...
Embodiment 2
[0056] Example 2 Optimization of PCR reaction conditions
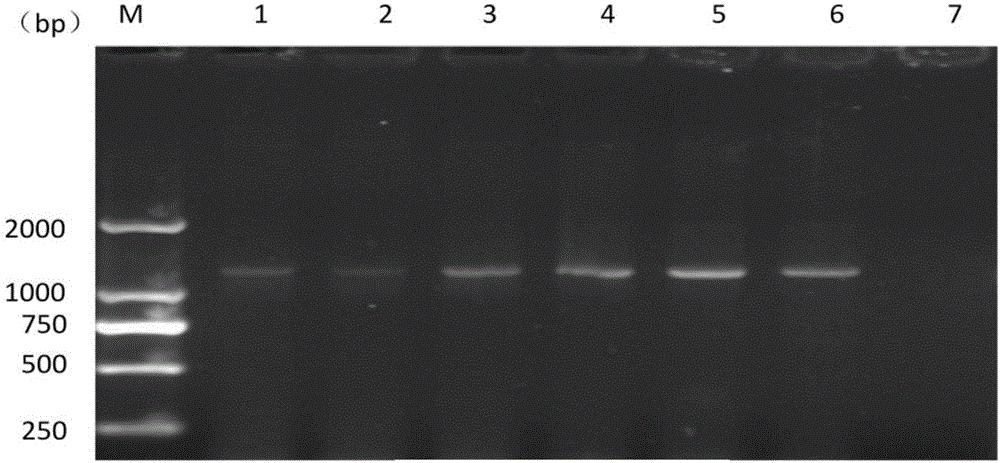
[0057] PCR amplification was carried out with primer 3 obtained by screening in Example 1. The PCR reaction system was the same as that in Example 1. The annealing temperature was adjusted between 50°C and 65°C and the number of cycles was adjusted between 30 and 45. The results showed that: GRAF -3 / GRAR-3 is the best annealing temperature for primer PCR at 65℃ ( figure 2 ); The number of cycles is 35.
Embodiment 3
[0058] Example 3 Composition of the kit
[0059] The kit composition of this embodiment includes: a sample DNA extraction solution, a PCR reaction solution and a positive control.
[0060] The sample DNA extraction solution here includes blood and semen DNA extraction solutions packaged independently; tissue DNA extraction solutions and water samples, and stool DNA extraction solutions.
[0061] Among them, the composition of blood and semen DNA extract is: 250 mL red blood cell lysate (10 mL DDT) for 50 reactions, 15 mL Buffer GA, 15 mL Buffer GB, 13 mL Buffer GD, 15 mL Buffer PW, 15 mL Buffer TE, 1 mL proteinase K, 10 mL 96% to 100% alcohol.
[0062] The composition of the tissue DNA extraction solution is: 50 mL nuclear lysate for 50 reactions, 30 mL 0.5M EDTA, 50 mL Wizard @ SV Lysis Buffer, 185mL column cleaning solution, 25mL RNase A solution, 2×25mL nuclease-free water.
[0063] The composition of water sample and stool DNA extraction solution is: 140 mL of ASL Buffer for 50 r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com