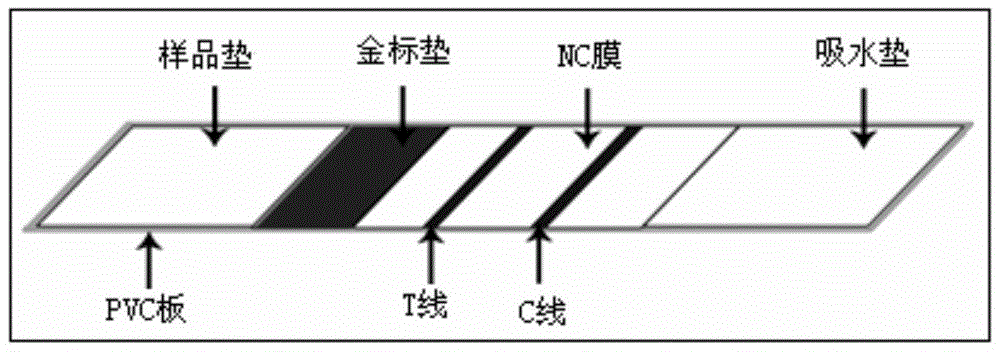
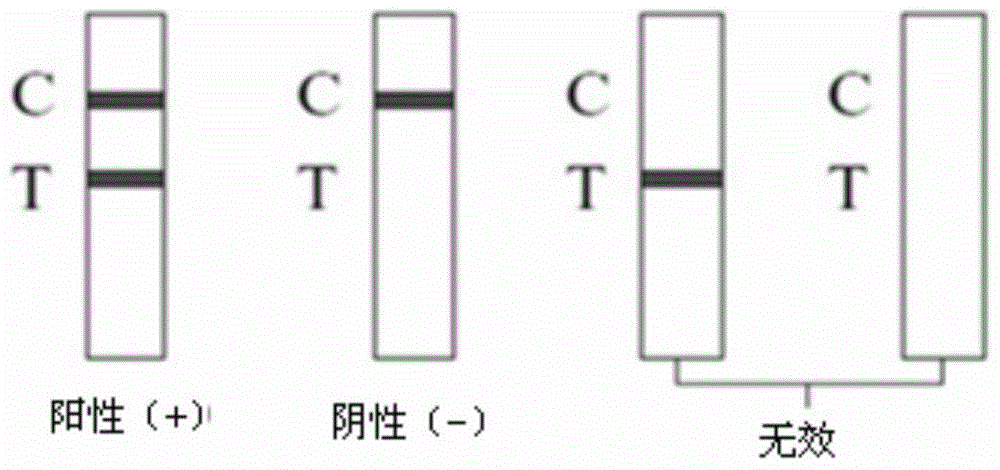
Toxoplasma detection kit
A detection kit and Toxoplasma gondii technology, applied in the biological field, can solve the problems of unsuitable on-site detection and long-term detection, and achieve the effects of easy promotion, high sensitivity and large expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment one kit preparation
[0027] The kit of the present invention comprises: test strips, standard products, reference products, and sample diluent; wherein the standard product is Toxoplasma gondii positive serum, the reference product is Toxoplasma gondii negative serum, and the sample diluent is 10mM, pH7.5 Phosphate buffered saline (PB), consisting of NaH 2 PO 4 ·H 2 O1.38g, Na 2 HPO 4 12H 2 O3.58g, add double distilled water to 100ml, adjust the pH to 7.5.
[0028] The kit of the present invention is prepared through the following steps:
[0029] (1) Preparation of anti-SAG3 monoclonal antibody: 8-10 weeks old BALB / c mice were taken, and each mouse was intraperitoneally injected with 1 mL of paraffin oil; 7 days later, each mouse was intraperitoneally injected with 1 x 10 6 A monoclonal hybridoma cell line 4D5 (preserved in the China Center for Type Culture Collection, preservation number CCTCCNO: C201624, preservation date: 2016.2.1), ascites was co...
Embodiment 2
[0037] Purification of Example Secondary Anti-Toxoplasma gondii SAG3 Monoclonal Antibody
[0038] 1. Materials
[0039] Monoclonal antibody ascites was preserved by our laboratory; reagents such as ammonium sulfate and n-octanoic acid were of domestic analytical grade; the freeze dryer and microplate reader were purchased from Thermo Company; the centrifuge was purchased from HITACHI.
[0040] 2. Monoclonal Antibody Purification
[0041] Monoclonal antibody was purified by octanoic acid-ammonium sulfate precipitation method. First, take out ascites from the refrigerator, put it on ice to melt, then centrifuge at 12000rpm for 30min, take the supernatant, and discard the precipitated impurities; mix the ascites and acetate buffer according to 1 : 2, mixed at room temperature according to 33μl / ml ascites, added n-octanoic acid drop by drop, stirred at room temperature for 30min, and overnight at 4°C to make it fully precipitated; under the condition of 4°C, centrifuged at 12000r...
Embodiment 3
[0046] Example 3 Performance Evaluation of Colloidal Gold Immunochromatography Rapid Detection Test Strip
[0047] 1. Materials
[0048] Toxoplasma gondii positive serum, Cryptosporidium pig positive serum, Streptococcus suis positive serum, Salmonella pig positive serum, Cysticercus pig positive serum, Coccidia pig positive serum, Eperythrozoon pig positive serum, Trichinella pig positive serum Toxoplasma gondii Natural whole protein, Toxoplasma gondii negative serum is preserved by our laboratory.
[0049] 2. Specificity test
[0050] The colloidal gold immunochromatographic rapid detection test strip detection method established in this study was used to detect porcine Toxoplasma gondii positive serum, Porcine Cryptosporidium positive serum, Streptococcus suis positive serum, Porcine Salmonella positive serum, Cysticercus porcine positive serum, porcine Coccidia positive serum, porcine Eperythrozoon positive serum, porcine Trichinella positive serum, in order to determine...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com