Preparation method of S-(+)-naproxen fatty acyl glyceride prodrug
A technology for prodrugs of fatty acylglycerols and esters, applied in the field of preparation of S--naproxen prodrugs, can solve the problems of harsh reaction conditions, many by-products, and no stereoselectivity, so as to reduce toxic and side effects and improve drug production. effect, and the effect of expanding the scope of drug safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
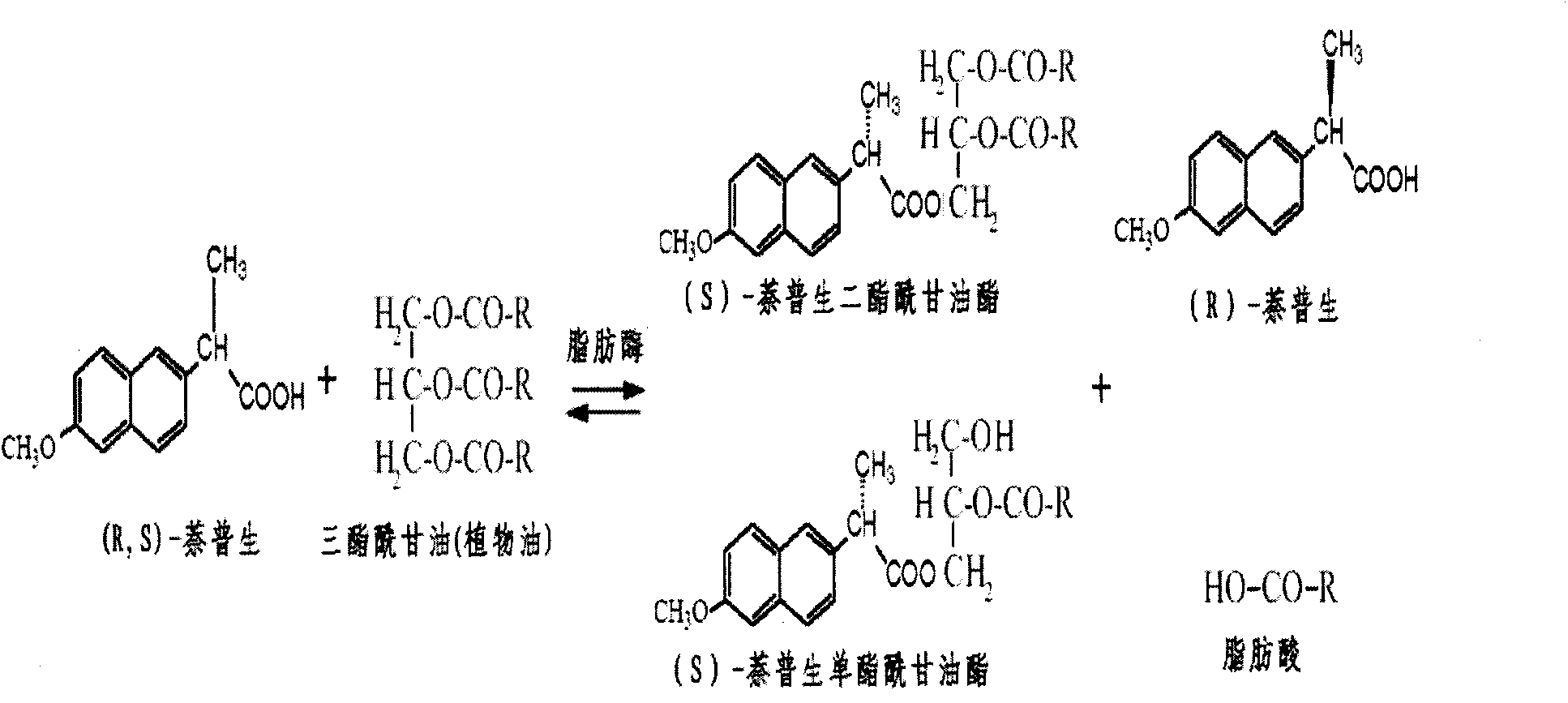
[0023] Specific embodiment one: the preparation method of the present embodiment S-(+)-naproxen fatty acylglyceride prodrug, carry out according to the following steps:
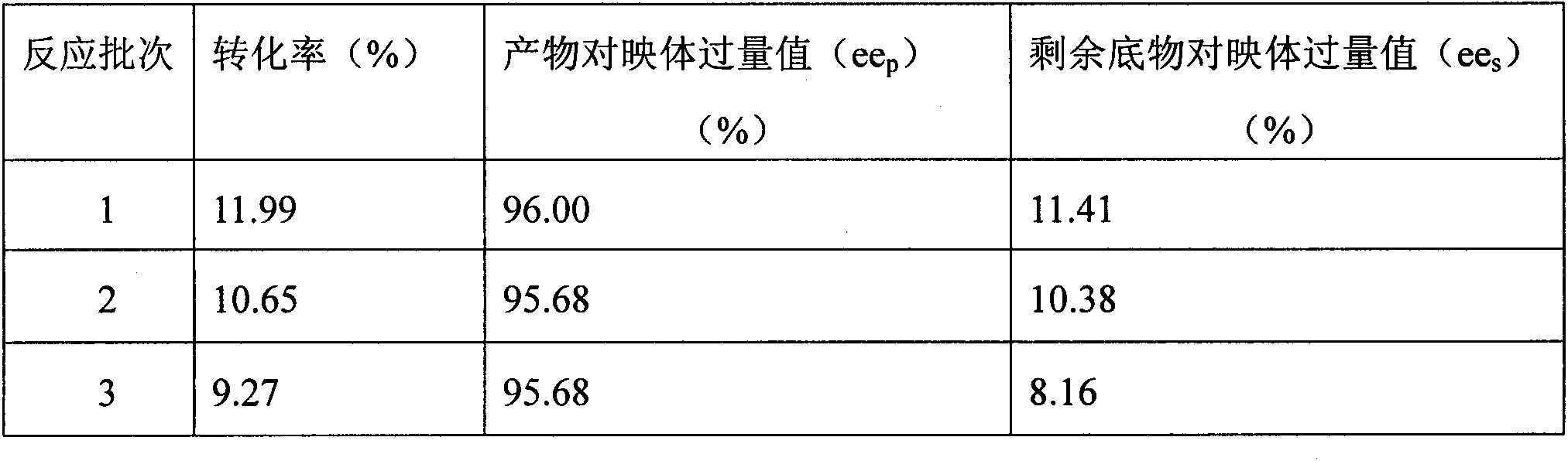
[0024] 1. Use molecular sieves to remove water from the vegetable oil so that the water activity is less than 0.01, then add (R, S)-naproxen to the dewatered vegetable oil, and use a homogenizer to homogenize (R, S)-naproxen Disperse in vegetable oil, add Candida columnar lipase, and then add molecular sieves, carry out enzyme-catalyzed reaction at 32°C and 300 rpm stirring conditions, and react for 3 to 6 days to obtain a mixture;
[0025] Two, adopt the method for centrifugation or filtration to remove solid, obtain oily liquid product, promptly complete the preparation of S-(+)-naproxen fatty acylglyceride prodrug; Wherein the substrate (R, S)-naproxen in step 1 The molar ratio of raw material and vegetable oil is 0.1-2:1; the mass ratio of candida cylindrica lipase and substrate (R, S)-naproxen is 1:5-10....
specific Embodiment approach 2
[0026] Embodiment 2: This embodiment differs from Embodiment 1 in that: in step 1, the molar ratio of the substrate (R, S)-naproxen to vegetable oil is 0.5-1.5:1. Others are the same as the first embodiment.
specific Embodiment approach 3
[0027] Embodiment 3: The difference between this embodiment and Embodiment 1 or 2 is that in step 1, the mass ratio of Candida cylindrica lipase to the substrate (R, S)-naproxen is 1:7-8. Others are the same as those in Embodiment 1 or 2.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap