Method for secretory production of protein
A protein and heterologous protein technology, applied in the field of coryneform bacteria, can solve problems such as unclear and reduced penicillin-binding protein relationships
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0225] Example 1: Construction of Corynebacterium glutamicum lacking penicillin-binding proteins PBP1a and PBP1b (1) Construction of vector pBSΔCgl0278 for deletion of PBP1a encoding gene Cgl0278
[0226] The genome sequence of Corynebacterium glutamicum ATCC13032 and the sequence of the Cgl0278 gene encoding the penicillin-binding protein PBP1a have been determined (Genbank Accession No. BA000036, NCBI Gene Entry NCgl0274). Referring to this sequence, primers shown as SEQ ID NO: 01, 02, 03, and 04 were synthesized. By PCR, using the chromosomal DNA of the Corynebacterium glutamicum ATCC13869 strain prepared in a conventional manner (the method of Saito and Miura [Biochim. Biophys. Acta, 72, 619 (1963)]) as a template, and primer SEQ ID NO:01 and 02, and SEQ ID NO:03 and 04 respectively amplified the about 1 kbp 5' side upstream region and about 1 kbp 3' side downstream region of the Cgl0278 gene encoding PBP1a. Then, by using the two amplified DNA fragments as templates and ...
Embodiment 2
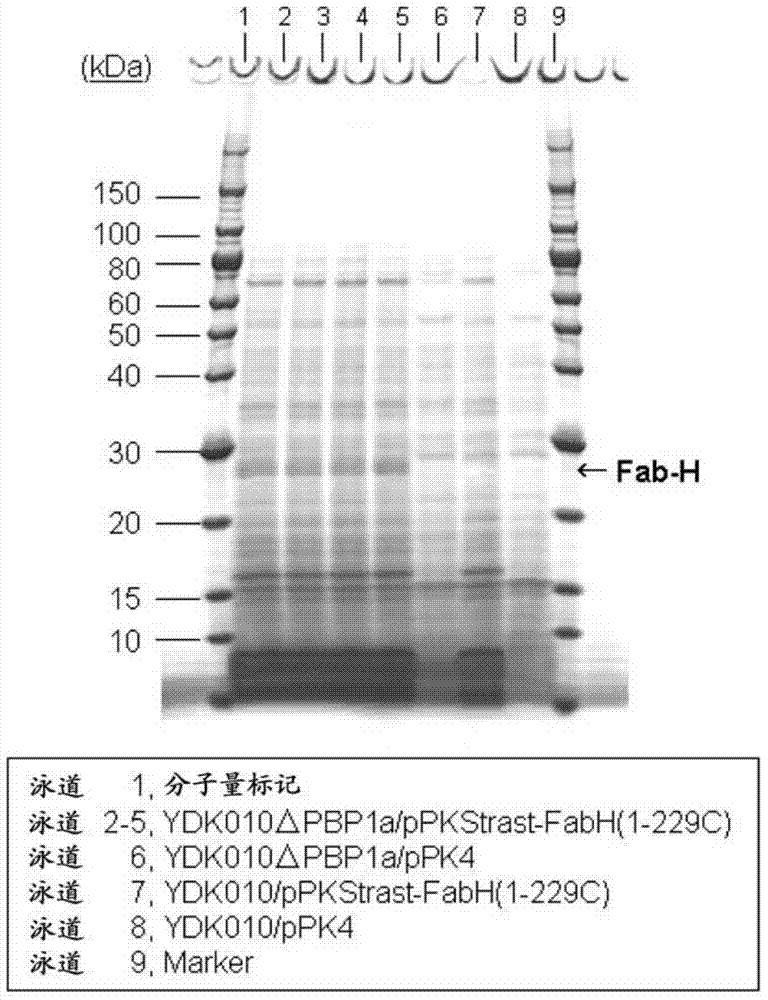
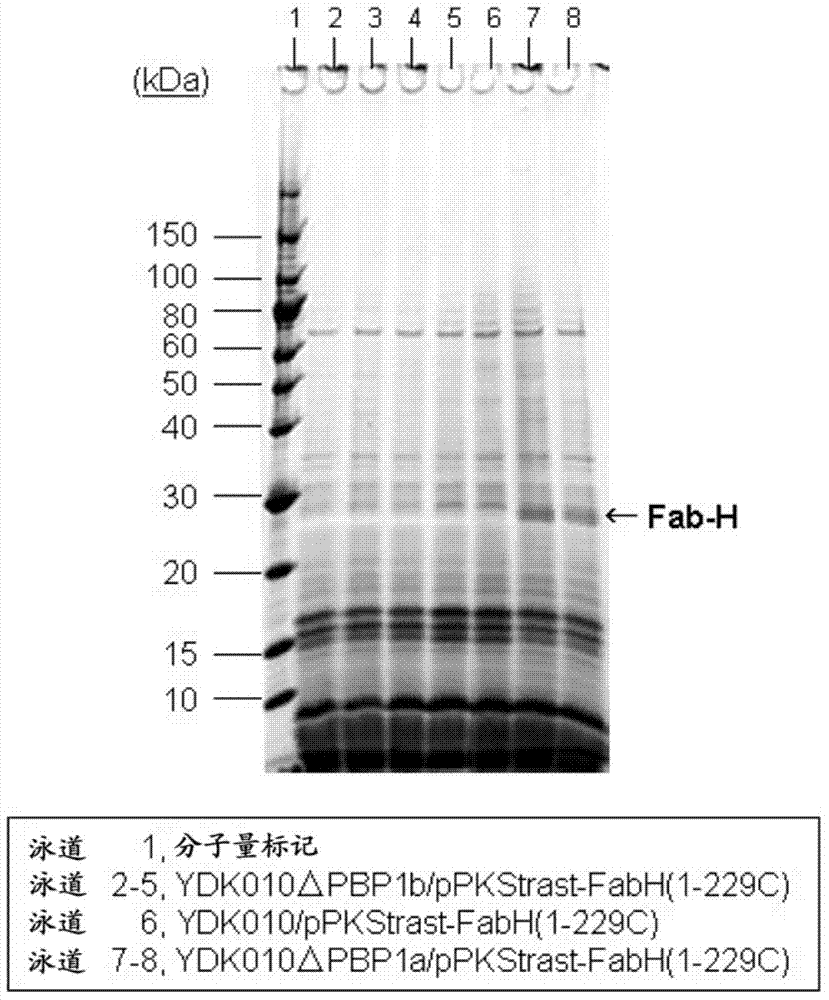
[0230] Then, the Corynebacterium glutamicum YDK010 strain described in WO2004 / 029254 was transformed with the constructed pBSΔCgl0278 and pBSΔCgl2986, respectively. Corynebacterium glutamicum YDK010 strain is a cell surface protein PS2-deficient strain of Corynebacterium glutamicum AJ12036 strain (FERM BP-734) (WO2004 / 029254). According to the methods described in WO2005 / 113744 and WO2006 / 057450, strains were screened from the obtained transformants to obtain Cgl0278 gene-deficient YDK010ΔPBP1a strain and Cgl2986 gene-deficient YDK010ΔPBP1b strain. Example 2: Using penicillin-binding protein PBP1a and PBP1b deficient Corynebacterium glutamicum strains to secrete and express the H chain region of antibody trastuzumab (trastuzumab) Fab fragment
[0231] (1) Construction of a plasmid for secreting and expressing the H chain region of the antibody trastuzumab Fab fragment
[0232] The gene sequence of the variable region of the H chain of the breast cancer cell-specific antibody ...
Embodiment 3
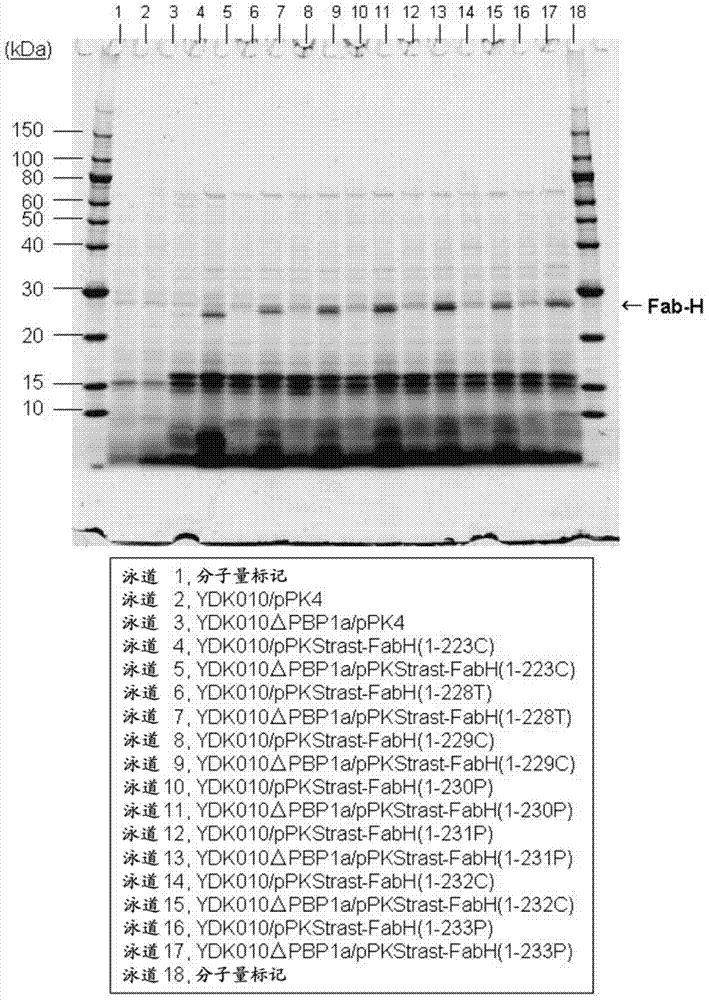
[0239] Example 3: Use of penicillin-binding protein PBP1a-deficient Corynebacterium glutamicum to secrete and express the L chain region of the antibody trastuzumab Fab fragment
[0240] (1) Construction of a plasmid for secreting and expressing the L chain region of the antibody trastuzumab Fab fragment
[0241] The gene sequence of the variable region of the L chain of the breast cancer cell-specific antibody trastuzumab has been determined (Genbank Accession No. AY513485). Referring to this sequence and the sequence of the non-variable region of the L chain of the universal antibody, considering the codon usage frequency of Corynebacterium glutamicum, DNAs shown as SEQ ID NO: 55-70 were synthesized. Using the above-mentioned DNA as a template, and using the DNA sequences synthesized respectively as shown in SEQ ID NO:71 and 72 as primers, the full-length L chain region of trastuzumab was amplified by PCR to obtain a DNA sequence as shown in SEQ ID NO: A DNA fragment of abo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com