Reproducible kidney cancer therapeutic DNA vaccine
A DNA vaccine and replication-type technology, applied in gene therapy, pharmaceutical formulations, anti-tumor drugs, etc., can solve problems such as reproducible DNA vaccines that have not yet appeared, achieve enhanced induction of efficient specific cellular immunity and humoral immunity, and improve Effects of immune potency and large storage space
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
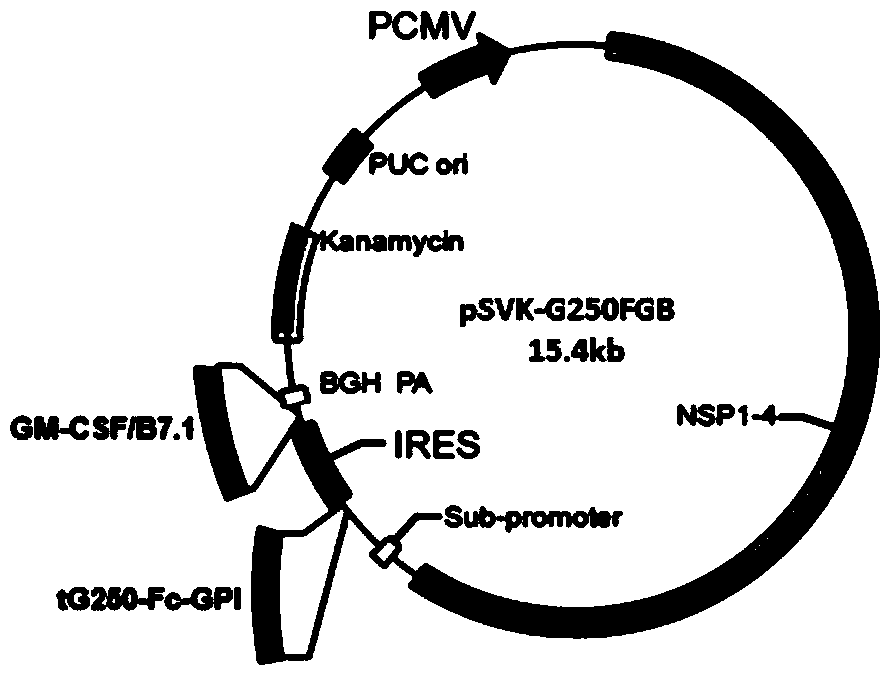
[0058] Example 1. Construction and expression of replicable renal cancer therapeutic DNA vaccine pSVK-G250FGB
[0059] 1. Construction of a replicable DNA vaccine vector
[0060] 1. Construction of the recombinant vector pUC19-PS-KANA carrying PS and KANA fusion gene (PS-KANA)
[0061] The construction method of the recombinant vector pUC19-PS-KANA is as follows:
[0062] 1) Amplify the PS gene: use the replicon DNA vaccine vector pSCA1 containing the ampicillin resistance gene (see the literature Yu YZ, Sun ZW, Yu W Y. Chinese Journal of Biotechnology, 2005, 21(5): 33- for the construction method 38) as template, in primer PSCA-F
[0063] (5’-CCGTCTAGAGATCATAATCAGCCAT-3’) and PSCA-R
[0064] (5'-CCGGCATGCCTCGAGACTAGTCTGTCAGACCAAG-3') guide PCR amplification of the flanking sequence of the ampicillin resistance gene, the 5'flanking sequence contains the restriction endonuclease Xba I recognition site, the 3'end The flanking sequence contains the restriction enzyme Sph I recognition sit...
Embodiment 2
[0122] Example 2. Construction of a renca mouse cell line stably expressing human G250 and a human G250 mouse kidney cancer tumor-bearing model
[0123] Because it is necessary to stably express human G250 mouse kidney cancer model in the research process of kidney cancer vaccine or immunotherapy targeting G250, the research work of this part of the experiment is to establish a renca mouse cell line stably expressing human G250 and human G250 mouse kidney cancer tumor-bearing model. The human G250 gene was amplified by PCR, and inserted into the pIRES-neo eukaryotic expression vector according to the DNA recombination technology to obtain the recombinant expression plasmid pIRES-neo-G250. The plasmid was stably transfected into renca mouse cells by cationic liposome-mediated method. Positive clones were selected by adjusting the concentration of G418. Western blotting and immunofluorescence tests verified that the human G250 gene was transfected into mouse renca cell lines. Usin...
Embodiment 3
[0178] Example 3. Detection of tumor suppressor activity and immunological mechanism of the replicable renal cancer therapeutic DNA vaccine pSVK-G250FGB
[0179] In Example 3, Figure 13-22 The plasmid used for the immunization of mice in the middle E group is marked as pSVK-sig-tG250-Fc-GPI-IRES-GM / B7 and the replicable renal cancer therapeutic DNA vaccine pSVK-G250FGB is the same plasmid, but the name is simple and complicated. The differences are hereby explained.
[0180] The renal cancer therapeutic DNA vaccine pSVK-G250FGB constructed in Example 1 was delivered by intramuscular injection plus electroporation to immunize the tumor-bearing mice obtained in Example 2. By observing and recording the tumor growth of mice in each group, the tumor suppressor activity of the replicable DNA vaccine pSVK-G250FGB was studied. The ELISA method was used to detect the specific antibody titers in the serum of immunized mice, the Elispot method was used to detect the activation of lymphocy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com