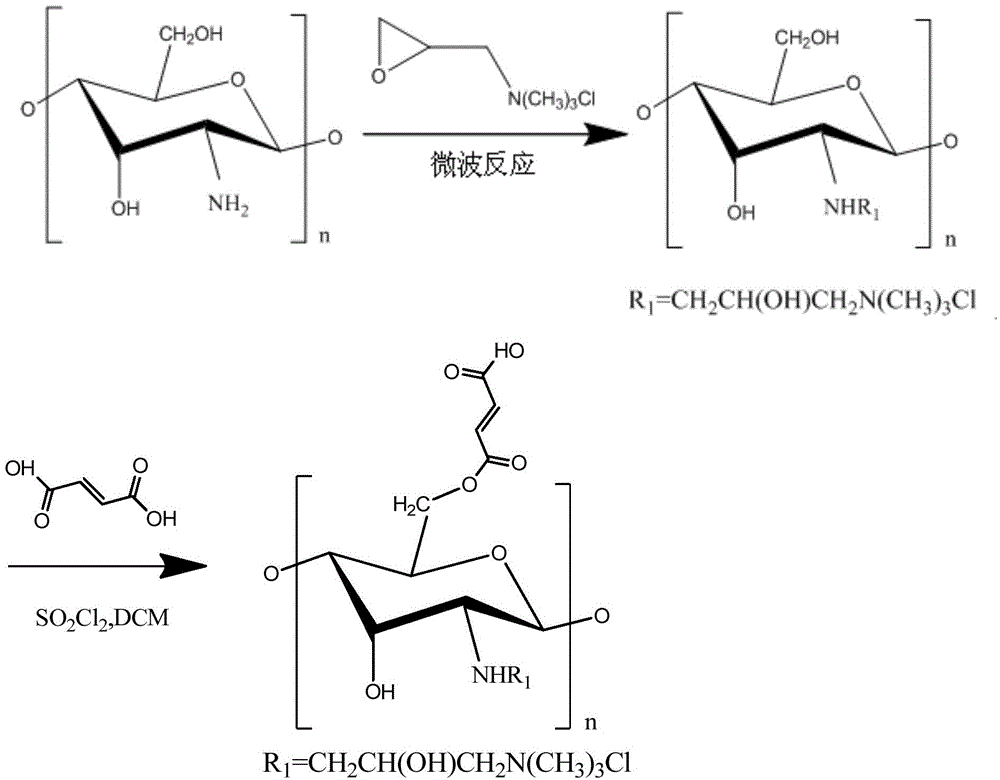
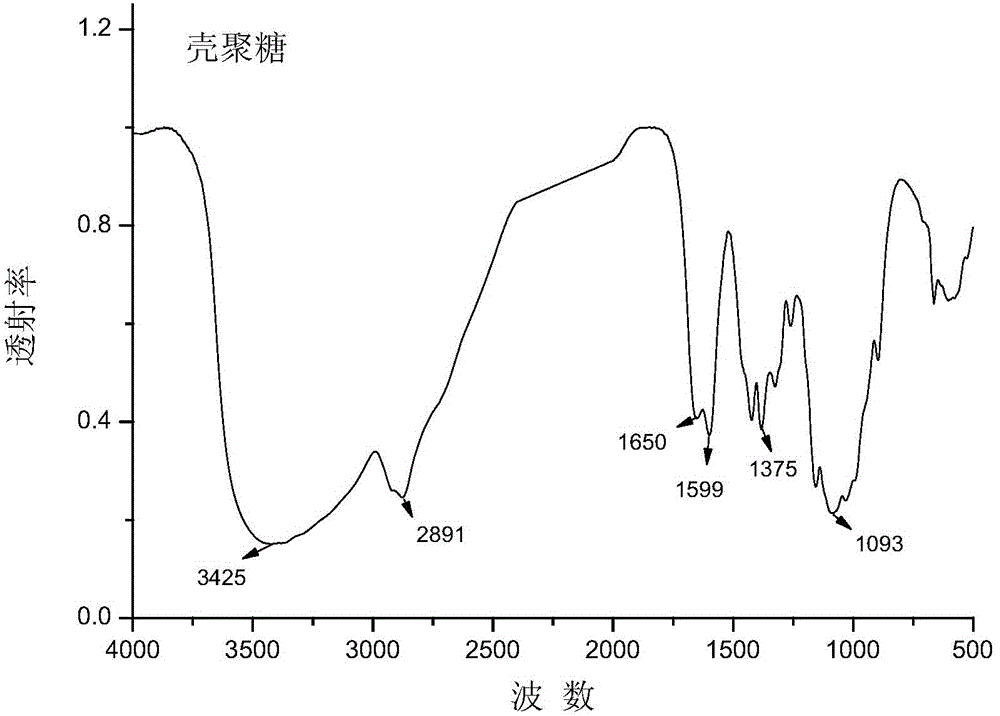
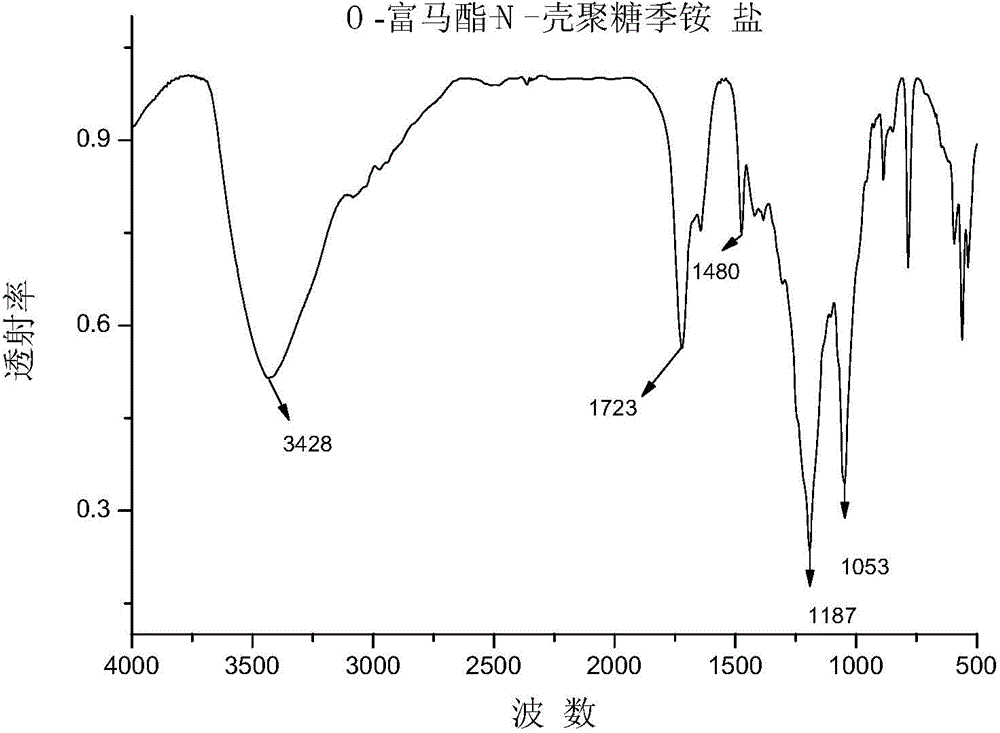
O-fumarate ester-N-chitosan quaternary ammonium salt and preparation method and application thereof
A technology of chitosan quaternary ammonium salt and fumarate, which is applied in the field of functional derivative O-fumarate-N-chitosan quaternary ammonium salt and its preparation, can solve the problems of limiting antibacterial applications and achieve enhanced antibacterial Activity and hydrophilicity, good antibacterial properties, and the effect of expanding the range of applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] A kind of O-fumarate-N-chitosan quaternary ammonium salt, its preparation method is as follows:
[0033] (1) Add 3g of a viscosity-average molecular weight of 5.0×10 to a three-necked flask 4 , chitosan with a deacetylation degree of 95%, add 50mL of isopropanol, stir and make it swell for 0.5h, then add 25mL of 40wt% sodium hydroxide solution, stir for 6h to make chitosan alkaline at 25°C Form a swellable alkalization center under the conditions, then add 9g of 2,3-epoxypropyltrimethylammonium chloride in several times, transfer to a microwave reaction instrument and microwave for 120min under the condition of 40°C and 600W , washed and filtered with 95% (v / v) ethanol solution, added acetone for precipitation, and dried under vacuum at 60° C. to obtain N-chitosan quaternary ammonium salt.
[0034] The degree of substitution of N-chitosan quaternary ammonium salt was measured to be 61.4% by potentiometric titration.
[0035] (2) Take 1.16g of fumaric acid and disperse...
Embodiment 2
[0038] A kind of O-fumarate-N-chitosan quaternary ammonium salt, its preparation method is as follows:
[0039] (1) Add 3g of a viscosity-average molecular weight of 5.0×10 to a three-necked flask 4 , chitosan with a deacetylation degree of 95%, add 50mL of isopropanol, stir and make it swell for 0.5h, then add 25mL of 40wt% sodium hydroxide solution, stir for 6h to make chitosan alkaline at 25°C Form a swollen alkalization center under the conditions, then add 15g of 2,3-epoxypropyltrimethylammonium chloride several times, transfer to a microwave reaction instrument, and microwave for 90min under the condition of 50°C and 600W , washed and filtered with 95% (v / v) ethanol solution, added acetone for precipitation, and dried under vacuum at 60° C. to obtain N-chitosan quaternary ammonium salt.
[0040] The degree of substitution of the N-chitosan quaternary ammonium salt was measured to be 69% by potentiometric titration.
[0041] (2) Disperse 2.32g of fumaric acid in 10mL of...
Embodiment 3
[0044] A kind of O-fumarate-N-chitosan quaternary ammonium salt, its preparation method is as follows:
[0045] (1) Add 3g of a viscosity-average molecular weight of 5.0×10 to a three-necked flask 4 , chitosan with a deacetylation degree of 95%, add 50mL of isopropanol, stir and make it swell for 0.5h, then add 25mL of 40wt% sodium hydroxide solution, stir for 6h to make chitosan alkaline at 25°C Form a swellable alkalization center under the conditions, then add 21g of 2,3-epoxypropyltrimethylammonium chloride in several times, transfer to a microwave reaction instrument and microwave for 60min at 70°C and 700W , washed and filtered with 95% (v / v) ethanol solution, added acetone for precipitation, and dried under vacuum at 60° C. to obtain N-chitosan quaternary ammonium salt.
[0046] The degree of substitution of the N-chitosan quaternary ammonium salt was measured to be 78% by potentiometric titration.
[0047] (2) Take 3.48g of fumaric acid and disperse it in 10mL of dic...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of substitution | aaaaa | aaaaa |
| degree of substitution | aaaaa | aaaaa |
| degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com