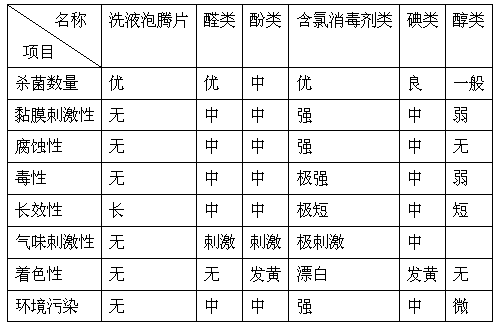
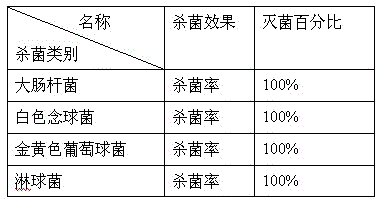
A kind of lotion effervescent tablet and preparation method thereof
A technology of effervescent tablet and lotion, which is applied in the direction of medical formula, medical preparations containing active ingredients, organic active ingredients, etc. It can solve the problems of shortened storage time of medicine effect, inconvenient carrying, unsatisfactory effect of gynecological inflammation, etc. , to achieve the effect of prolonging the storage time of the drug effect, broad spectrum of bactericidal and bacteriostatic effects, and easy to carry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] 1. Weigh raw materials
[0045] Based on a batch of 1000 tablets, weigh 36g of chlorhexidine acetate, 90g of polyhexamethylene biguanide, 3g of vitamin C, 500g of sodium bicarbonate, and 900g of tartaric acid as the main ingredients. For later use, weigh 1g of crospovidone, 35g Povidone K30, 15g soluble starch, 70g polyethylene glycol 6000, 100g adipic acid, 50g carboxymethyl starch sodium as auxiliary materials, for subsequent use;
[0046] 2. Crushing
[0047] Polyethylene glycol 6000 and adipic acid are respectively pulverized into 100 mesh fine powder with a universal pulverizer, and set aside;
[0048] 3. Premix
[0049] Sodium bicarbonate, vitamin C, chlorhexidine acetate and soluble starch were put into a high-efficiency mixer and mixed for 2 minutes to obtain a mixture;
[0050] 4. Soft materials
[0051] Adding a mass percent concentration of 5% povidone K30 solution formulated with ethanol to the mixed material, the volume concentration of the ethanol is 9...
Embodiment 2
[0069] 1. Weigh raw materials
[0070] Based on a batch of 1000 tablets, weigh 90g of chlorhexidine acetate, 36g of polyhexamethylene biguanide, 7g of vitamin C, 900g of sodium bicarbonate, and 500g of tartaric acid as the main ingredients. For later use, weigh 3g of crospovidone, 50g Povidone K30, 8g soluble starch, 20g polyethylene glycol 6000, 176g adipic acid, 10g carboxymethyl starch sodium as auxiliary materials, for subsequent use;
[0071] 2. Crushing
[0072] Polyethylene glycol 6000 and adipic acid are respectively pulverized into 100 mesh fine powder with a universal pulverizer, and set aside;
[0073] 3. Premix
[0074] Sodium bicarbonate, vitamin C, chlorhexidine acetate and soluble starch were put into a high-efficiency mixer and mixed for 7 minutes to obtain a mixture;
[0075] 4. Soft materials
[0076] Adding ethanol to the mixed material with a mass percentage concentration of 5% povidone K30 solution, the volume concentration of the ethanol is 95%, and m...
Embodiment 3
[0088] 1. Weigh raw materials
[0089] Based on a batch of 1000 tablets, weigh 50g of chlorhexidine acetate, 50g of polyhexamethylene biguanide, 5g of vitamin C, 641g of sodium bicarbonate, and 640g of tartaric acid as the main ingredients. For later use, weigh 2g of crospovidone, 20g Povidone K30, 12g soluble starch, 50g polyethylene glycol 6000, 300g adipic acid, 30g carboxymethyl starch sodium as auxiliary materials, for subsequent use;
[0090] 2. Crushing
[0091] Polyethylene glycol 6000 and adipic acid are respectively pulverized into 100 mesh fine powder with a universal pulverizer, and set aside;
[0092] 3. Premix
[0093] Sodium bicarbonate, vitamin C, chlorhexidine acetate and soluble starch were put into a high-efficiency mixer and mixed for 5 minutes to obtain a mixture;
[0094] 4. Soft materials
[0095] Adding ethanol to the mixed material with a mass percentage concentration of 5% povidone K30 solution, the volume concentration of the ethanol is 95%, and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com