Reinforced tuberculosis subunit vaccine
A subunit vaccine and enhanced technology, applied in the direction of bacterial antigen components, antibody medical components, antibacterial drugs, etc., can solve problems such as unsatisfactory needs, and achieve the effect of improving immune protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
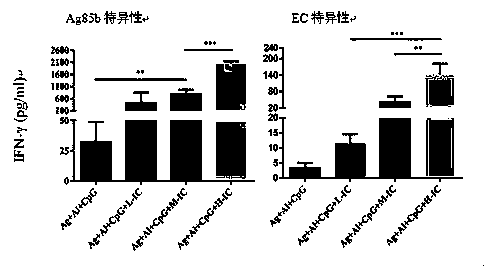
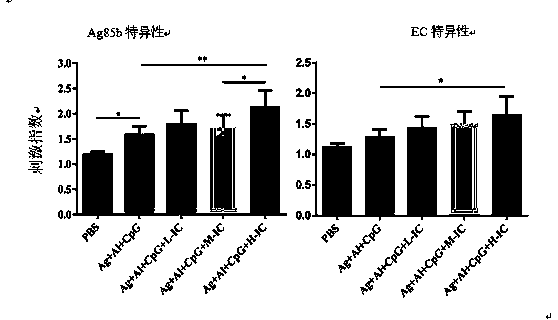
[0028] Example 1 Immunoprotective effect test
[0029] Materials and methods
[0030] 1. Experimental materials
[0031] 1. Mice and guinea pigs
[0032] Specific pathogen free (SPF) grade BALB / c mice (female, 6-8 weeks old) and SPF grade Hartley guinea pigs (half male and female, 300-400g) were purchased from the Experimental Animal Resource Center of the China Institute for Food and Drug Control. supply. Experimental animal use license number: scxk (Beijing) 2009-0017. During the experiment, they were kept in a negative pressure animal room that reached biosafety level III.
[0033] Reagent
[0034] Recombinant Mycobacterium tuberculosis protein Ag85b and recombinant Mycobacterium tuberculosis ESAT6-CFP10 fusion protein (EC) were prepared according to the method disclosed in patent document CN103386128A, BCG-CPG (prepared according to the method disclosed in Chinese patent 200410033878.1), and improved Roche medium, BCG The vaccine was produced by the Chengdu Institute...
Embodiment 2
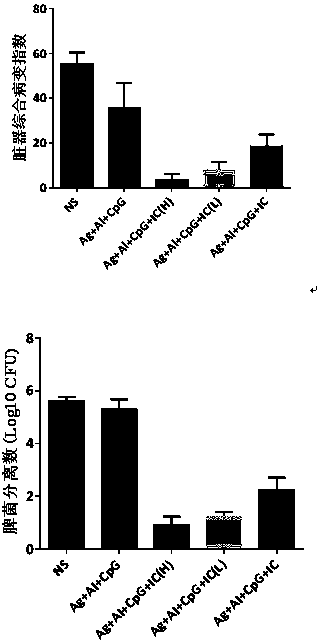
[0061] Example 2 Immunotherapy Experiment of Guinea Pig Latent Infection Model
[0062] In view of the above results, the present invention evaluates Ag+Al+CpG+PolyI:C on the latent infection model of guinea pigs, that is, Ag85b (10 μg)+EC (10 μg)+Al(OH)3 (0.2mg)+CpG (75 μg)+ Immunotherapeutic effect of Poly I:C (50 μg) vaccine.
[0063] 1. Methods and steps
[0064] 1. Grouping and Immunization of Guinea Pigs
[0065] Table 2 Experimental groups of guinea pigs
[0066]
[0067] Note: EC is ESAT6-CFP10
[0068] 40 SPF grade Hartley guinea pigs (300-350g / guinea pig), half male and half male, were divided into 5 groups with 8 guinea pigs in each group. The guinea pigs were challenged subcutaneously with 5.0×10 3 After CFU Mtb, intragastric administration of isoniazid, 5 mg / cattle, 3 times a week, for 4 weeks in total. Then each group was injected with the corresponding vaccine according to the above table for treatment, a total of 6 injections, with an interval of 2 wee...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com