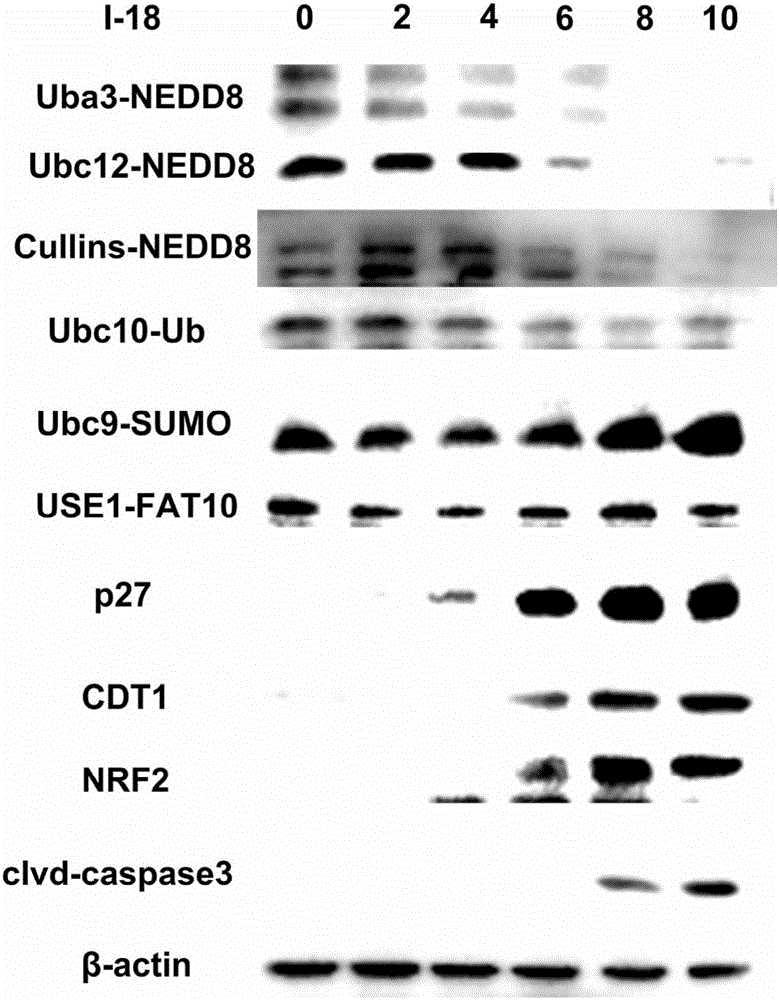
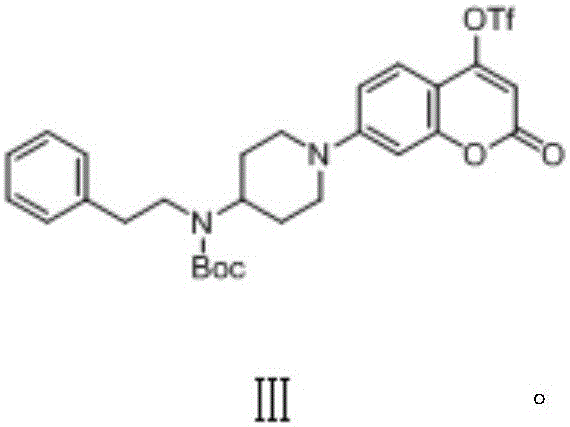
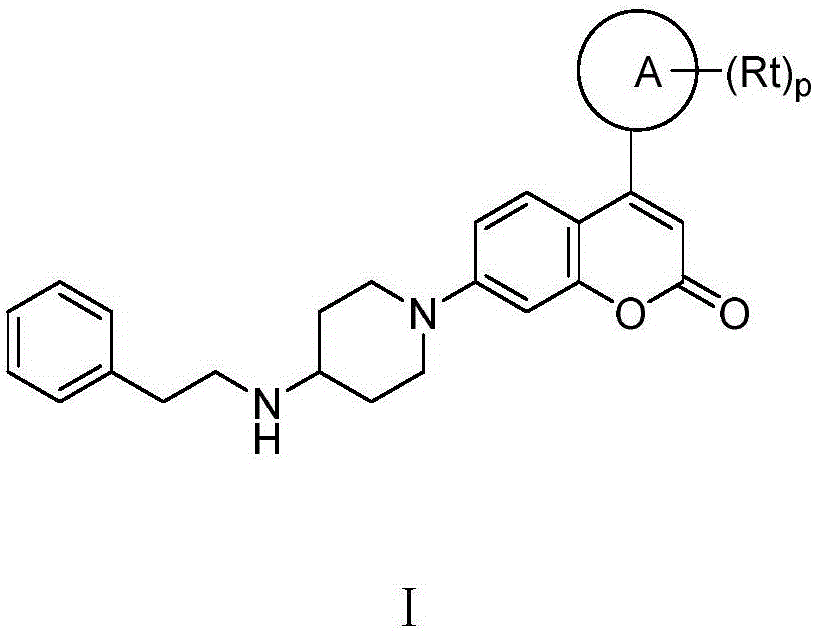
Coumarins NEDD8 activating enzyme inhibitor
A technology of coumarins and inhibitors, applied in the field of biochemistry, can solve problems such as the lack of similar products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0127] Example 1 7-(4-(phenethylamino)piperidine-1-substituted)-4-phenyl-coumarin (I-1)
[0128] Steps:
[0129] (1) Synthesis of methyl 1-(4-bromo-2-hydroxy-phenyl)pyruvate (1)
[0130] Add 1000mL of anhydrous toluene and 31g of NaH into a 2L four-neck round bottom flask, and stir in an ice bath for 5min under nitrogen protection. 400 mL of a toluene solution dissolved with 60 g of 4-bromo-2-hydroxyacetophenone was added dropwise under ice-bath conditions. After the dropwise addition, continue to stir in an ice bath for 20 min. Then 67 g of diethyl carbonate were added dropwise. After the dropwise addition was completed, it was heated to reflux and stirred overnight. After the reaction was detected by TLC, the temperature was cooled in an ice bath, and the pH was adjusted to acidity with concentrated hydrochloric acid. After filtration, the filter cake was washed with ethyl acetate, and the filtrate was concentrated to the crude product. The product 60.41 g was purified...
Embodiment 2
[0143] Example 2 7-(4-phenethylaminopiperidine-1-substituted)-4-(3-fluoro-4-methylbenzene-1-substituted)coumarin (I-2)
[0144] The synthesis method is as in Example 1, except that the raw materials used in step (7) are 0.147g intermediate 7, 0.042g 3-fluoro-4-methylphenylboronic acid, 0.014g tetrakis(triphenylphosphine) palladium and 0.061g Sodium carbonate, 74.6 mg was obtained, the yield was 81.2%, and the purity was 99.0%.
[0145] HNMR(300MHz,DMSO-d6)δ:7.45(t,J=7.6Hz,H),7.23-7.38(m,8H),6.95(d,2H),6.09(s,H),4.05(d,J =12.8Hz,2H),3.40(s,H),3.23(s,2.65),2.90(m,4H),2.33(s,3H),2.08(d,J=10.7Hz,2H),1.51(q ,J=11.8Hz,2H).CNMR(500MHz,DMSO-d6)δ:161.93,160.74,159.99,156.39,154.27,152.96,137.52,135.27,135.20,132.52,132.48,129.09,129.06,1227.82 ,126.22,124.71,115.54,115.36,111.93,109.81,109.09,101.15.
Embodiment 3
[0146] Example 3 7-(4-phenethylaminopiperidine-1-substituted)-4-(3-methyl-4-fluorobenzene-1-substituted)coumarin (I-3)
[0147] The synthesis method is as in Example 1, except that the raw materials used in step (7) are 0.16g intermediate 7, 0.045g 3-methyl-4-fluorophenylboronic acid, 0.015g tetrakis(triphenylphosphine) palladium and 0.067g Sodium carbonate, 64.9 mg was obtained, the yield was 56.5%, and the purity was 97.5%.
[0148] HNMR(300MHz,DMSO-d6)δ:7.44(d,J=7.3Hz,H),7.23-7.38(m,8H),6.96(d,2H),6.06(s,H),4.05(d,J =13.3Hz,2H),3.39(H),3.22(s,2H),2.90(m,4H),2.31(s,3H),2.08(d,J=11.0Hz,2H),1.51(q,J =11.6Hz,2H).CNMR(500MHz,DMSO-d6)δ:162.74,160.78,156.37,154.79,152.92,137.50,132.21,131.79,129.10,129.06,128.39,128.33,125.98,1121.25,2 ,115.76,111.92,109.69,109.27,101.13,54.66,45.87,45.19,32.22,27.51,14.58.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com