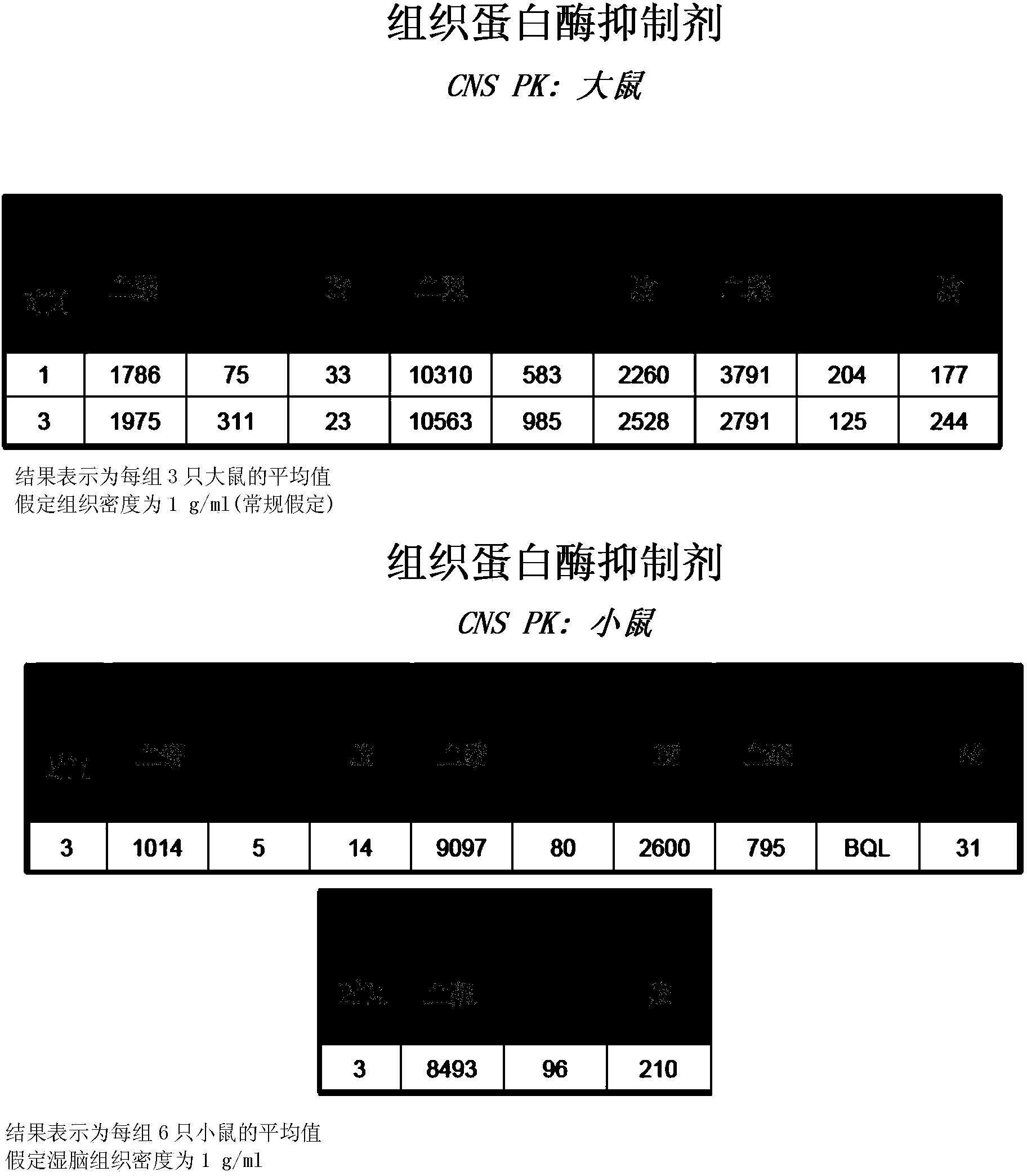
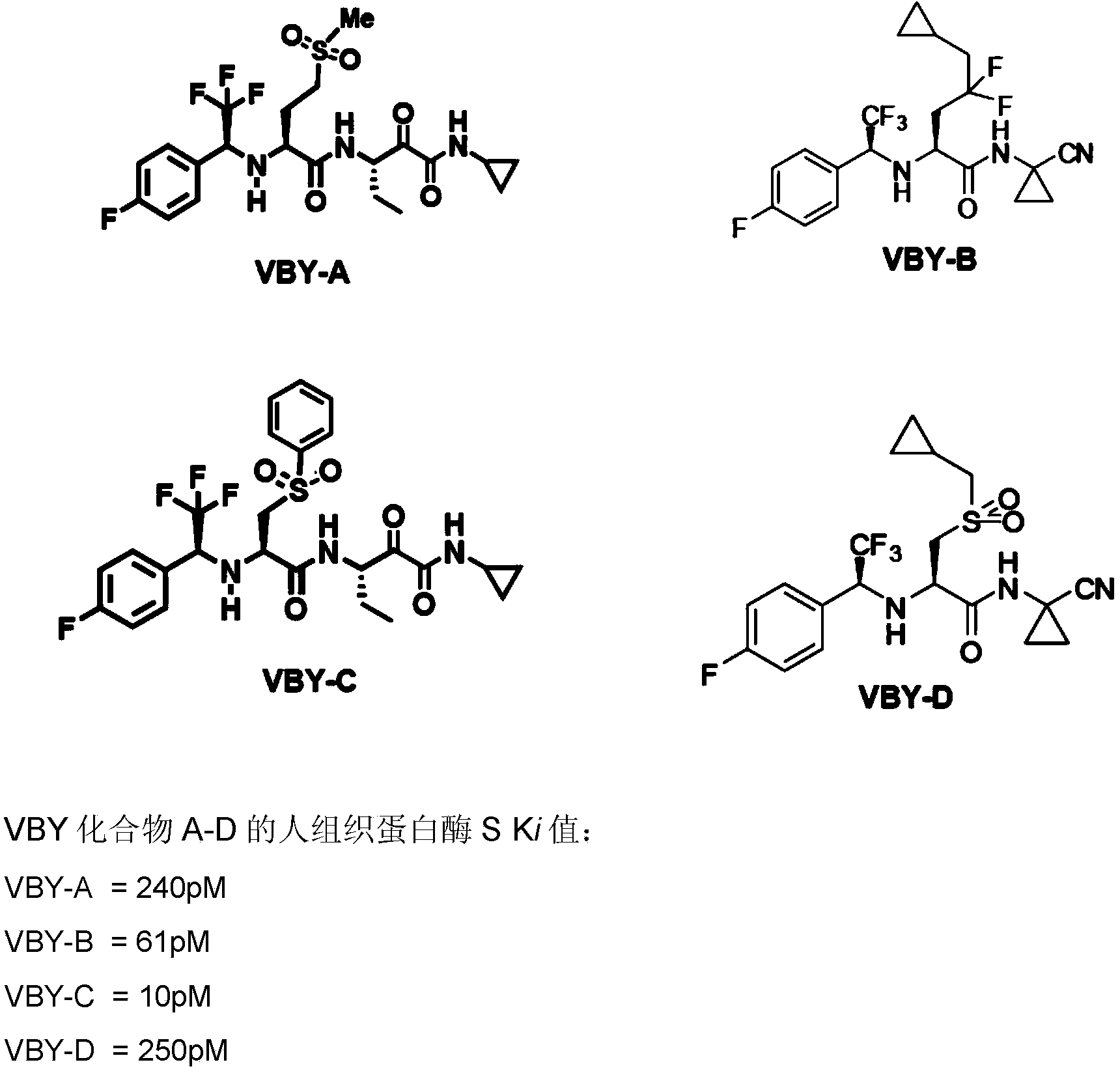
Cathepsin inhibitors for treating microglia-mediated neuron loss in the central nervous system
A technology of glial cells and neurons, applied in the field of cathepsin inhibitors for the treatment of microglial cell-mediated neuronal loss in the central nervous system
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
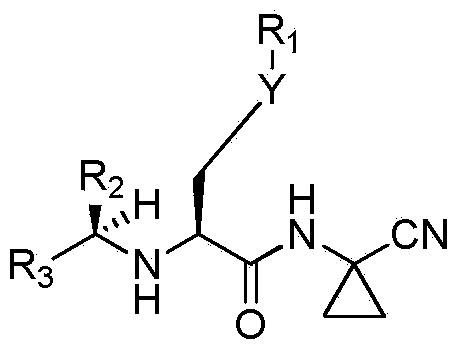
[0079] I. the present invention preferably uses certain compounds shown in formula (I) in the maximum range listed in the summary of the invention, for example:
[0080] (A) A group of preferred compounds used in the present invention are as follows:
[0081] R 1 is cycloalkylalkyl, phenylalkyl, or pyridylalkyl or aralkyl, wherein any aromatic ring or aromatic ring is optionally substituted by 1 or 2 substituents selected from the group consisting of halogen, lower Alkyl, CF 3 , lower alkoxy, or OCF 3 ;
[0082] R 3 is H or phenyl optionally substituted by 1 or 2 substituents selected from the group consisting of halogen, lower alkyl, CF 3 , lower alkoxy, or OCF 3 .
[0083] (B) In another preferred compound used in the present invention,
[0084] R 1 is alkyl, cycloalkylalkyl, phenylalkyl, or pyridylalkyl or aralkyl, wherein any aromatic ring or aromatic ring is optionally replaced by 1 or 2 substituents selected from the following group Substitution: halogen, lower...
Embodiment 1
[0122] N-(1-cyanocyclopropyl)-3-pyridin-3-ylmethylsulfonyl-2(R)-(2,2,2-trifluoro-1(S)-4-fluorophenylethylamine ) Synthesis of Propionamide
[0123]
[0124] step 1
[0125] At 0°C, in a nitrogen atmosphere, in 2(R)-[2,2,2-trifluoro-1(S)-(4-fluorophenyl)ethylamino]-3-trityl-sulfur Alkyl (sulfanyl) propionic acid (539mg, 1mmol, 90% de value, calculated by the purity of the trifluoromethyl group) CH 2 Cl 2 Trifluoroacetic acid (0.4 mL, 4 mmol) and triethylsilane (0.4 mL, 2 mmol) were added to the solution. The reaction mixture was stirred at room temperature for 2 h. The solvent was removed under reduced pressure and the residue was dissolved with 1N NaOH (12 mL). The aqueous layer was washed with hexane, and dioxane (12 mL) was added to the alkaline solution, P(CH 2 CH 2 COOH) 3 , HCl (28mg, 0.1mmol) and 3-chloromethyl-pyridine (196mg, 1.2mmol), the reaction mixture was stirred at room temperature for 2h. Dioxane was removed under reduced pressure and the residue was...
Embodiment 2
[0131] N-(1-cyanocyclopropyl)-2(R)-[2,2,2-trifluoro-1(S)-(2,4-difluoro-phenyl)ethyl-amino]-3 -Synthesis of (cyclopropylmethylsulfonyl) propionamide (compound 43)
[0132]
[0133] step 1
[0134] 2,4-Difluorobenzaldehyde (1.1 mL, 10.0 mmol) and (trifluoromethyl)trimethylsilane (1.77 mL, 12.0 mmol) were dissolved in THF (25 mL), cooled to 0°C. A solution of 1M TBAF in THF (76 μL, 76 μmol) was added to the above mixture, and the reaction mixture was warmed to room temperature. After 3.25 h, 2.5M HCl (25 mL) was added. After the reaction was stirred for 1 h, it was extracted with ether, and the organic layer was washed with brine and washed with Na 2 SO 4 dry. A racemic mixture of 2,2,2-trifluoro-1-(2,4-difluoro-phenyl)ethanol (2.5 g) was obtained after removal of the solvent under reduced pressure.
[0135] step 2
[0136] 2,2,2-Trifluoro-1-(2,4-difluorophenyl)ethanol (1.36g, 6.4mmol) was dissolved in dichloromethane (25mL), diisopropylethylamine (DIPEA, 5mL) was added...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com