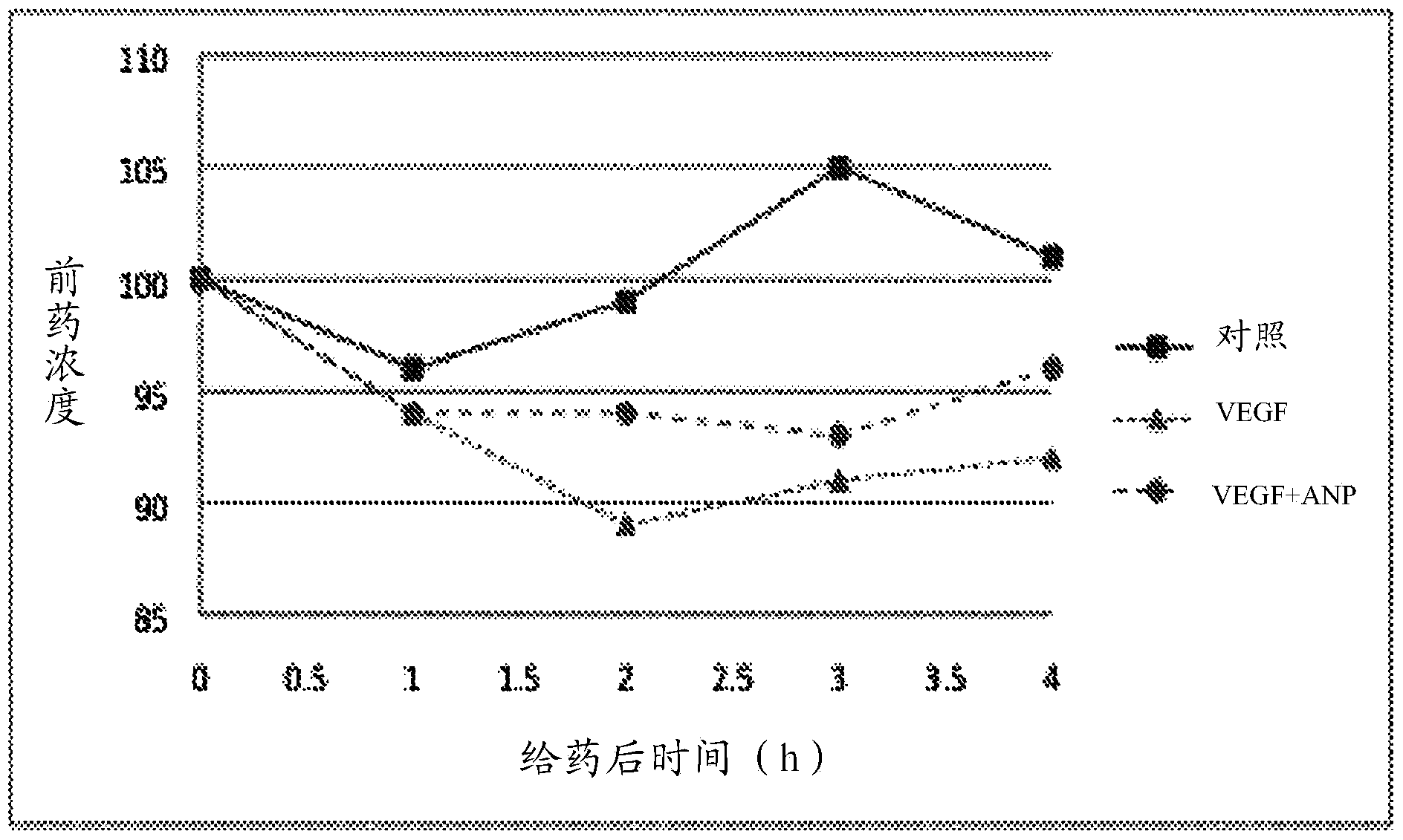
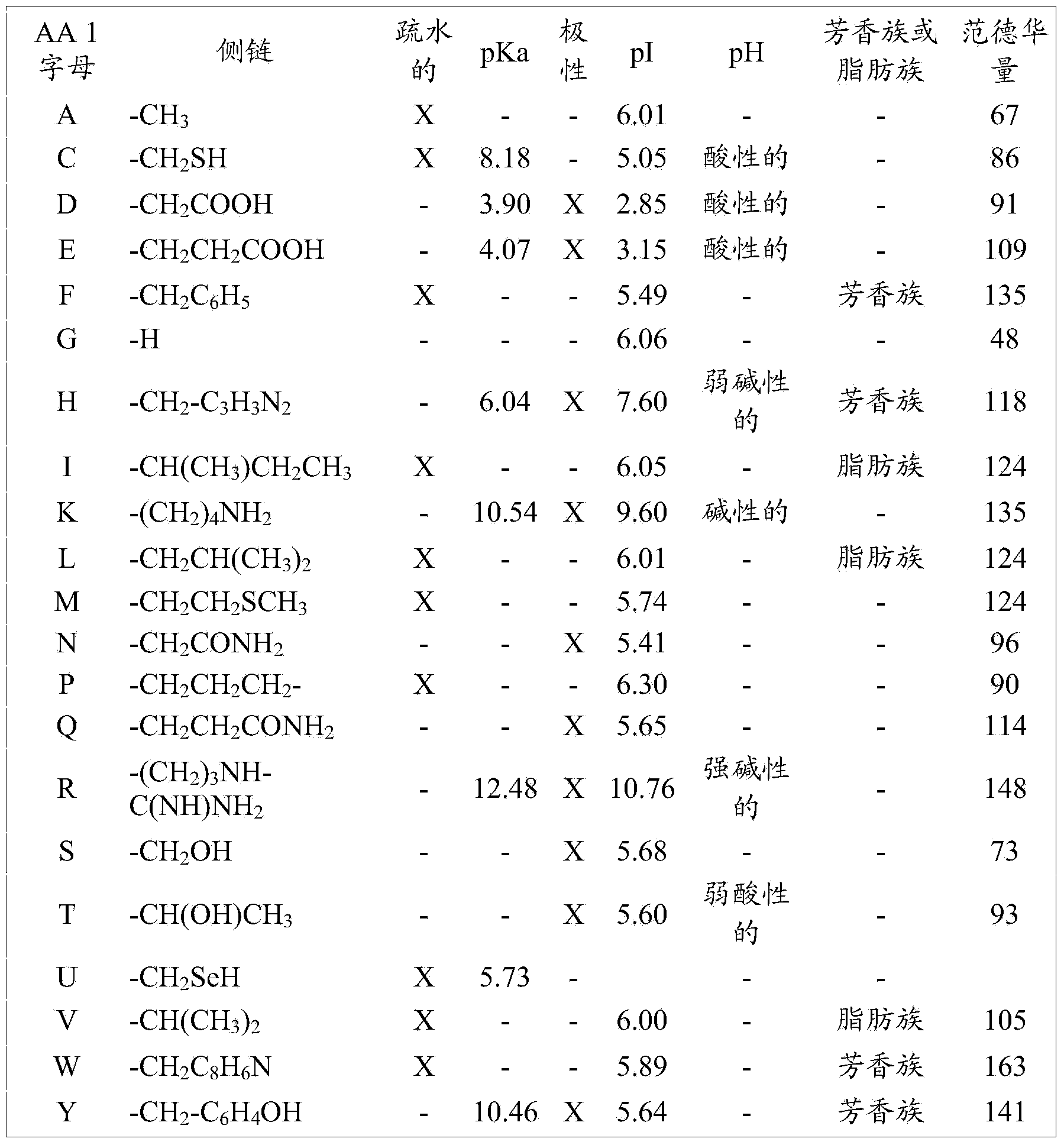
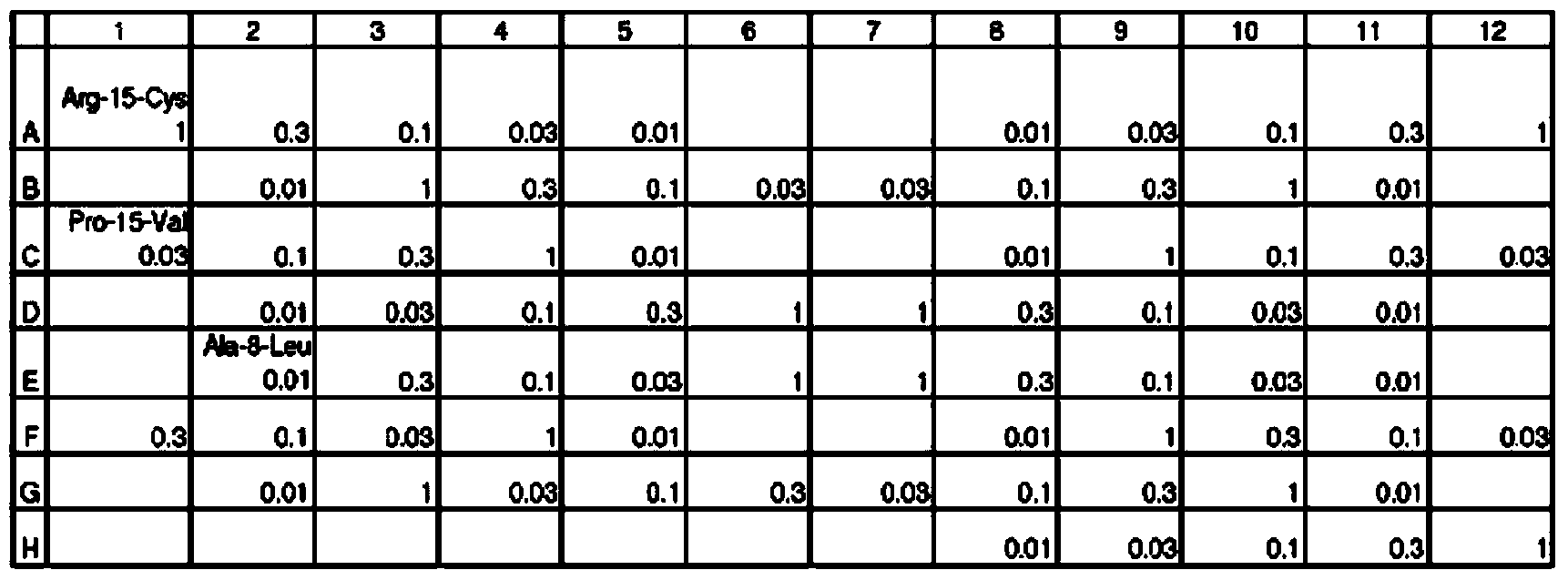
Methods and uses of anp (atrial natriuretic peptide), bnp (brain natriuretic peptide) and cnp (c-type natriuretic peptide)-related peptides and derivatives thereof for treatment of retinal disorders and diseases
A technology for retinal diseases, applied in the field of treatment of retinal diseases and diseases, can solve problems such as lack of cardiovascular function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0139] This example describes a general method for preparing peptides, proteins, fragments and mimetics that include conserved motifs.
[0140] Desired peptide fragments can be chemically synthesized. Alternative methods include the production of peptide fragments by enzymatic digestion, eg, by treating the protein with an enzyme that cleaves the protein at sites defined by specific amino acid residues, thereby producing peptide fragments. Another alternative approach involves genetic methods. Such techniques include introducing all or part of the gene encoding the compound into host cells, such as mammalian cells, HeLa cells or Cos cells or E. coli. Such host cells can express full-length or fragments, e.g., single-chain antibodies (see, e.g., Whitlow et al., In: Methods: A Companion to Methods in Enzymology 2:97 (1991), Bird et al., Science 242:423 (1988); and US Patent No. 4,946,778). For example, DNA encoding a peptide compound is digested with an appropriate restric...
Embodiment 2
[0143] This example describes data showing activity of two exemplary peptides with conserved motifs. Also included in this example is a description of an exemplary cell-based assay for determining anti-cell proliferative activity.
[0144] Human pancreatic cancer cell (HPAC) cells were obtained from ATCC and contained 2.5mM glutamine, 2mg / mL insulin, 5ug / mL transferrin, 1ng / mL EGF (epidermal growth factor), plus 5% FBS (fetal bovine Serum) in Dulbecco's modified Eagle's medium and Ham's F12 medium (DME-F12) (1:1). Cell culture at 37 °C in a 100% humidity atmosphere with 5% CO 2 cultivated in. Cells were routinely brought to confluence in T-flasks, rinsed with phosphate-buffered saline (PBS), and trypsinized for 5 minutes at room temperature. Trypsinized cells were rinsed from the plate with 10 ml medium and counted. The viability of subcultures is generally ≥95%.
[0145] Approximately 10,000 cells in 100 uL of medium were placed in each well of a 96-well plate and cultur...
Embodiment 3
[0156] This example describes the activity of peptides with conserved motifs and conserved amino acids predicted to function in the motifs (Table III). This example also describes additional exemplary peptide sequences with conserved motifs predicted to be active (Table IV).
[0157] Table III
[0158]
[0159] Positions 15 to 21 in Table III correspond to residues 1-8 of the conserved motif. Conserved amino acids for each of residues 1-8 include, for example, the following: (15) F, A, L; (16) G, K; (17) N, S, G, L, A; (18) any; (19) L, M; (20) S, D, R; (21) any; (22) I, L. Conserved amino acids for each of residues 1-8 include, for example, residue 1: hydrophobic, non-polar, non-ionizing residue; residue 2, non-hydrophobic (amphipathic or hydrophilic) residues; residue 3, non-ionized residues or residues with small side chains (0-2 carbon backbone with 3 and 4 weaker carbons); residue 4, any residue residue 5, a hydrophobic, nonpolar, nonionic, or aliphatic residue; re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com