Method both for preparation of iron oxide black and combined production of ammonium sulfate
A technology of iron oxide black and ammonium sulfate, applied in chemical instruments and methods, oxides of ferrous iron, iron oxide/iron hydroxide, etc., can solve the problem of poor tinting strength, long oxidation time and many by-products of iron oxide black and other problems, to achieve the effects of easy recycling, high processing costs, and environmental pollution problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
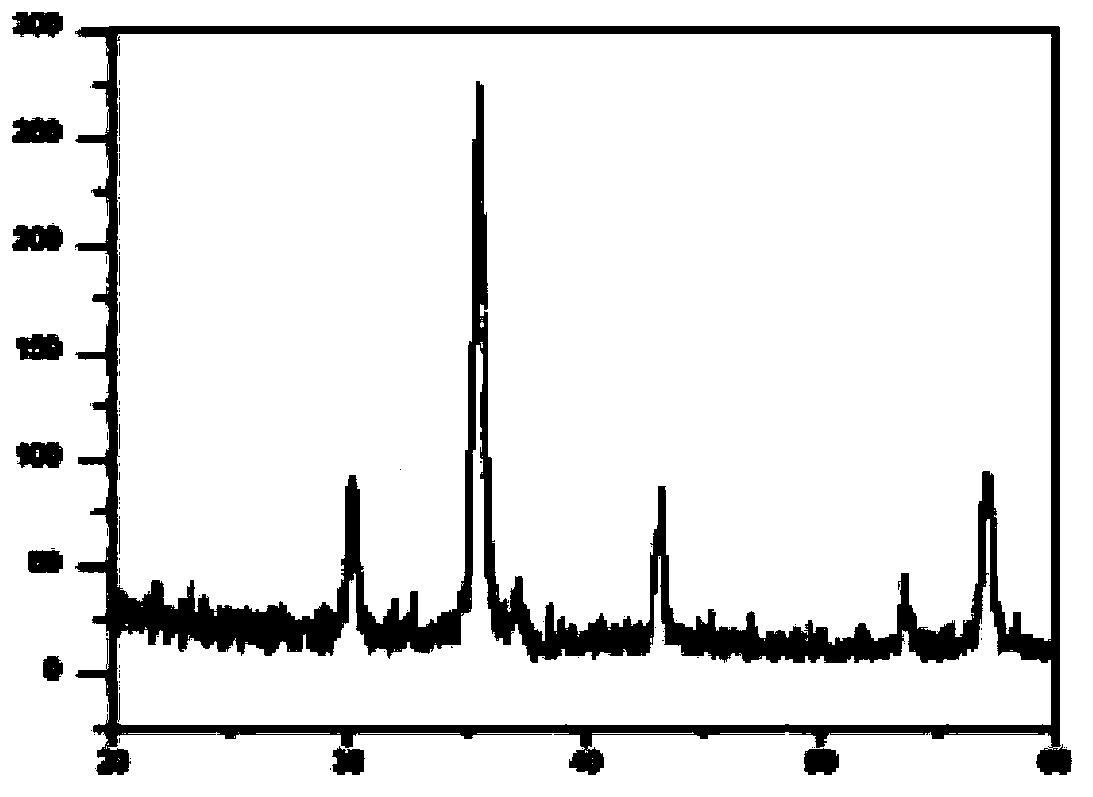
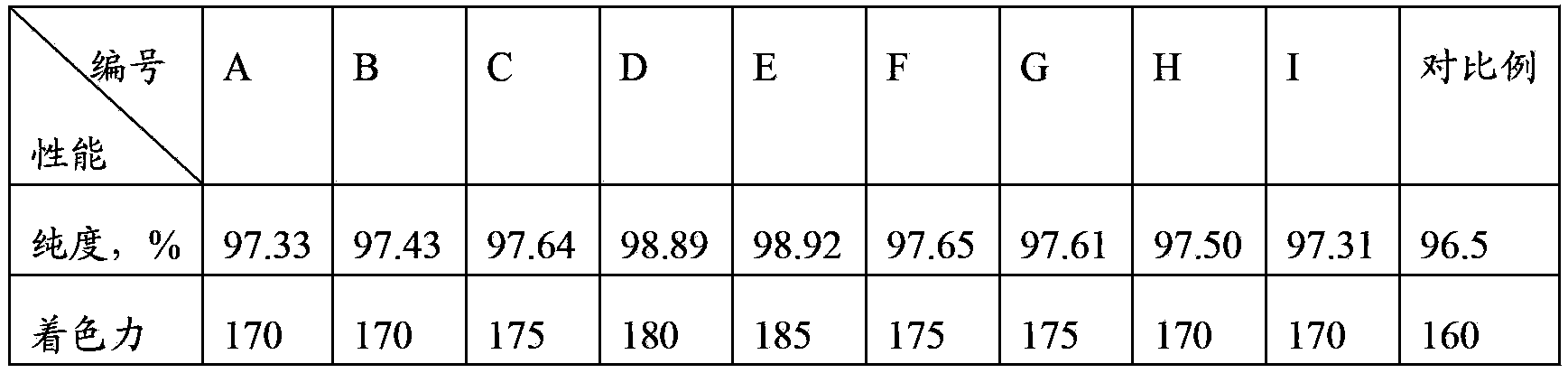
Image
Examples
Embodiment 1
[0024] (1) Mix 1.0kg of ferrous sulfate heptahydrate and 0.6kg of ammonium bicarbonate evenly, and knead at 20°C to complete the reaction;
[0025] (2) adding a certain amount of water to the reacted product in the step (1) to obtain a slurry with a concentration of 90 g / L;
[0026] (3) Introduce air into the slurry in step (2) at a rate of 6 L per minute, control the pH of the reaction system to 6.5 with ammonia water at a concentration of 22 wt%, and carry out the oxidation reaction at a temperature of 20°C ;
[0027] (4) After heating the slurry after the oxidation reaction in step (3) to 60°C, add 0.5kg of ferrous sulfate heptahydrate and 2.2kg of ammonia water with a concentration of 15w% to the slurry to perform an addition dehydration reaction ;
[0028] (5) After the reaction is completed, filter to obtain a filtrate with an ammonium sulfate concentration of 16.6wt% and a filter cake. The filtrate is used to recover ammonium sulfate, and the filter cake is dried at 6...
Embodiment 2
[0030] (1) Mix 1.2kg of ferrous sulfate heptahydrate and 0.7kg of ammonium bicarbonate evenly, and knead at 25°C to complete the reaction;
[0031] (2) adding a certain amount of water to the reacted product in step (1) to obtain a slurry with a concentration of 120 g / L;
[0032] (3) Introduce air into the slurry in step (2) at a rate of 7 L per minute, control the pH value of the reaction system to 7.0 with ammonia water with a concentration of 15 wt%, and carry out the oxidation reaction at a temperature of 25°C ;
[0033] (4) After the oxidation reaction slurry in the step (3) is heated to 65° C., 0.6 kg of ferrous sulfate heptahydrate and 3 kg of ammonia water with a concentration of 17 wt % are added to the slurry to perform an addition dehydration reaction;
[0034] (5) After the reaction is completed, filter to obtain a filtrate with an ammonium sulfate concentration of 15.5wt% and a filter cake. The filtrate is used to recover ammonium sulfate, and the filter cake is ...
Embodiment 3
[0036] (1) Mix 1.4kg of ferrous sulfate heptahydrate and 0.75kg of ammonium bicarbonate evenly, and knead at 30°C to complete the reaction;
[0037] (2) adding a certain amount of water to the reacted product in step (1) to obtain a slurry with a concentration of 140 g / L;
[0038] (3) Introduce air into the slurry in the step (2) at a rate of 8 L per minute, control the pH value of the reaction system to 7.5 with ammonia water with a concentration of 28 wt%, and carry out the oxidation reaction at a temperature of 30°C ;
[0039] (4) After the oxidation reaction slurry in the step (3) is heated to 70° C., 0.7 kg of ferrous sulfate heptahydrate and 3 kg of ammonia water with a concentration of 21 wt % are added to the slurry to perform an addition dehydration reaction;
[0040] (5) After the reaction is completed, filter to obtain a filtrate with an ammonium sulfate concentration of 17wt% and a filter cake. The filtrate is used to recover ammonium sulfate, and the filter cake ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com