A kind of preparation technology of iron oxide black co-producing ammonium sulfate
A technology for the preparation of iron oxide black, which is applied in the fields of ferrous oxide, iron oxide/hydroxide, inorganic chemistry, etc., and can solve the problems of poor tinting strength, many by-products, and long oxidation time of iron oxide black , to achieve strong coloring ability, reduce contact time, and solve the effect of high processing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
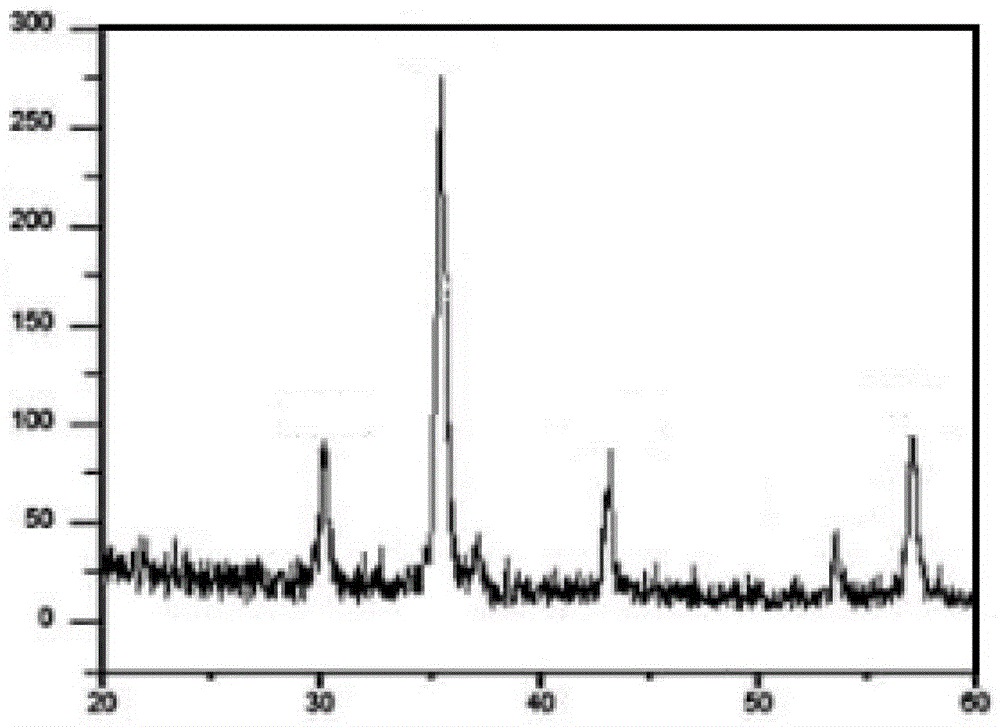
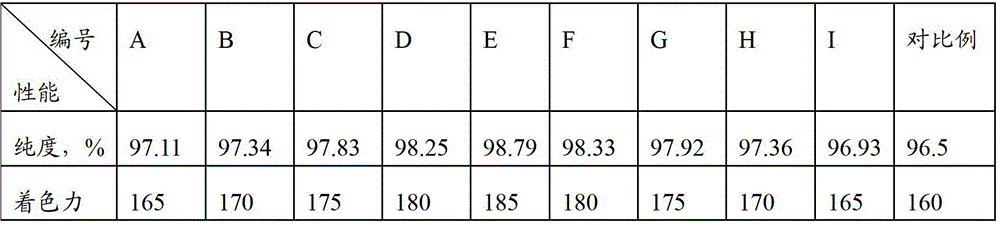
Image
Examples
Embodiment 1
[0023] Iron oxide black preparation method described in the present embodiment is as follows:
[0024] (1) Dissolve 1.5kg of ferrous sulfate heptahydrate in water to obtain ferrous sulfate solution;
[0025] (2) Add 8.7kg of ammonia water with a concentration of 15wt% to the ferrous sulfate solution in the step (1), and carry out neutralization reaction at a temperature of 20°C until the pH value of the solution is constant to 10;
[0026] (3) Warm up the solution after the neutralization reaction in step (2) to 80°C;
[0027] (4) Add 2.625kg of amorphous ferric oxyhydroxide to the heated solution in step (3), and use 28wt% ammonia water to control the pH value of the solution to 9 during the reaction;
[0028] (5) After the reaction is completed, filter to obtain a filtrate and a filter cake with an ammonium sulfate concentration of 7.12wt%. The filtrate is recovered from ammonium sulfate, and the filter cake is dried at 50° C. to prepare iron oxide black pigment A.
Embodiment 2
[0030] Iron oxide black preparation method described in the present embodiment is as follows:
[0031] (1) Dissolve 2.0kg of ferrous sulfate heptahydrate in water to obtain ferrous sulfate solution;
[0032] (2) Add 9.4kg of ammonia water with a concentration of 18wt% to the ferrous sulfate solution in the step (1), and carry out neutralization reaction at a temperature of 25°C until the pH value of the solution is constant at 10;
[0033] (3) Warm up the solution after the neutralization reaction in step (2) to 80°C;
[0034] (4) Add 3.0 kg of amorphous ferric oxyhydroxide to the heated solution in step (3), and use 28 wt% ammonia water to control the pH value of the solution to 9 during the reaction;
[0035] (5) After the reaction is completed, filter to obtain a filtrate with an ammonium sulfate concentration of 8.3wt% and a filter cake. The filtrate is subjected to recovery of ammonium sulfate, and the filter cake is dried at 60° C. to prepare iron oxide black pigment B....
Embodiment 3
[0037] Iron oxide black preparation method described in the present embodiment is as follows:
[0038] (1) Dissolve 2.3kg of ferrous sulfate heptahydrate in water to obtain ferrous sulfate solution;
[0039] (2) Add 11kg of ammonia water with a concentration of 20wt% to the ferrous sulfate solution in the step (1), and carry out neutralization reaction at a temperature of 30°C until the pH value of the solution is constant to 10;
[0040] (3) Warm up the solution after the neutralization reaction in step (2) to 85°C;
[0041] (4) Add 3.7kg of amorphous ferric oxyhydroxide to the heated solution in step (3), and use 28wt% ammonia water to control the pH value of the solution to 11 during the reaction;
[0042] (5) After the reaction is completed, filter to obtain a filtrate with an ammonium sulfate concentration of 8.4wt% and a filter cake. The filtrate is recovered from ammonium sulfate, and the filter cake is dried at 70° C. to prepare iron oxide black pigment C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| tinctorial strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com