Method for producing full-water-soluble magnesium ammonium nitrate by using steel belt granulator
A technology of ammonium magnesium nitrate and granulator, applied in the direction of magnesium nitrate, etc., can solve the problems of the purity of magnesium nitrate products that are easy to agglomerate, and achieve the effects of improving water solubility and content, improving poor water solubility, and enhancing strength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
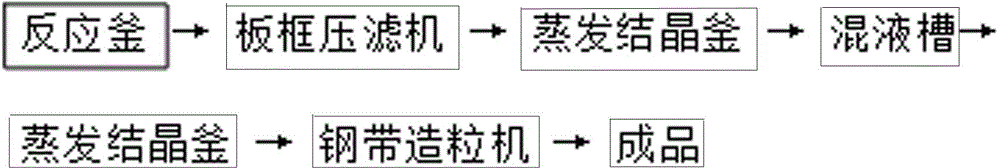
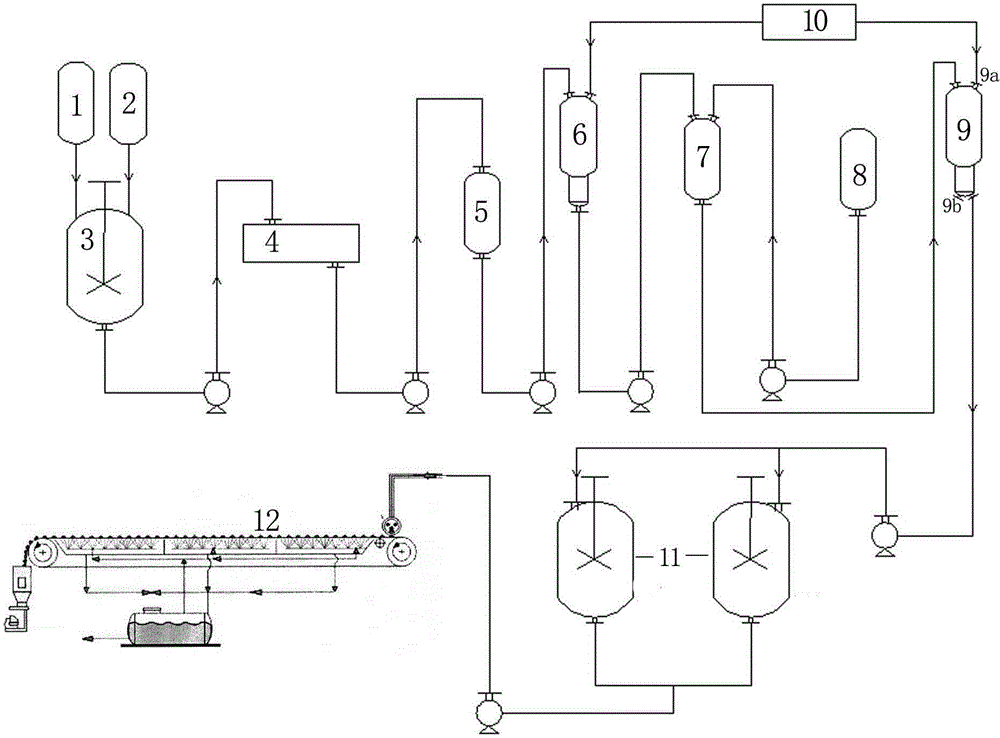
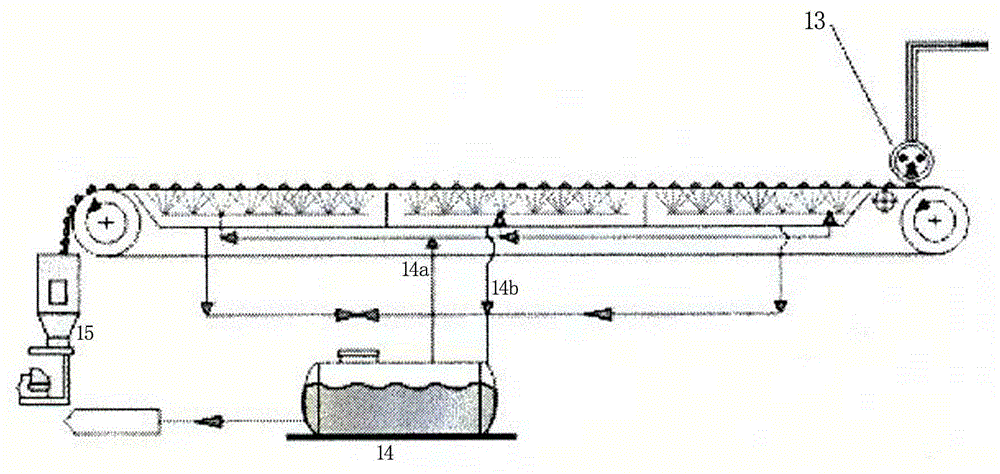
[0014] Example 1 A method for producing fully water-soluble magnesium ammonium nitrate using a steel belt granulator, comprising the following steps: (a) adding magnesium oxide and nitric acid to the reaction kettle at a molar ratio of 1:2 for neutralization reaction, the concentration of the nitric acid solution 40%, the temperature was controlled at 72°C and the reaction was stirred for 1.5 hours, and then the pH of the reaction solution was adjusted to 6.5 with magnesium oxide; (b) The magnesium nitrate neutralization solution obtained was filtered into a plate and frame filter press to obtain a transparent filtrate; (c ) pump the filtrate into the primary evaporator for evaporation and concentration, and evaporate to a specific gravity of 1.300g / cm 3 (d) adding ammonium nitrate solution to the concentrated magnesium nitrate solution, the ammonium nitrate solution accounts for 4.5% of the concentrated magnesium nitrate solution quality, and the concentration of the ammonium ...
Embodiment 2
[0017] Example 2 A method for producing fully water-soluble magnesium ammonium nitrate using a steel belt granulator, comprising the following steps: (a) adding magnesium oxide and nitric acid to the reaction kettle at a molar ratio of 1:2 for neutralization reaction, the concentration of the nitric acid solution 41%, the temperature was controlled at 76°C and the reaction was stirred for 1.7 hours, and then the pH of the reaction solution was adjusted to 7.0 with magnesium oxide; (b) The magnesium nitrate neutralization solution obtained was filtered into a plate and frame filter press to obtain a transparent filtrate; (c ) pump the filtrate into the primary evaporator for evaporation and concentration, and evaporate to a specific gravity of 1.080g / cm 3 (d) adding ammonium nitrate solution to the concentrated magnesium nitrate solution, the ammonium nitrate solution accounts for 5.5% of the concentrated magnesium nitrate solution quality, and the concentration of the ammonium ...
Embodiment 3
[0020] Example 3 A method for producing fully water-soluble magnesium ammonium nitrate using a steel belt granulator, comprising the following steps: (a) adding magnesium oxide and nitric acid to the reactor at a molar ratio of 1:2 for neutralization reaction, the concentration of the nitric acid solution 39%, the temperature was controlled at 78°C and the reaction was stirred for 1.6 hours, and then the pH of the reaction solution was adjusted to 7.5 with magnesium oxide; (b) The magnesium nitrate neutralization solution obtained was filtered into a plate and frame filter press to obtain a transparent filtrate; (c ) pump the filtrate into the primary evaporator for evaporation and concentration, and evaporate to a specific gravity of 1.200g / cm 3 (d) adding ammonium nitrate solution to the concentrated magnesium nitrate solution, the ammonium nitrate solution accounts for 3.5% of the concentrated magnesium nitrate solution quality, and the concentration of the ammonium nitrate ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com