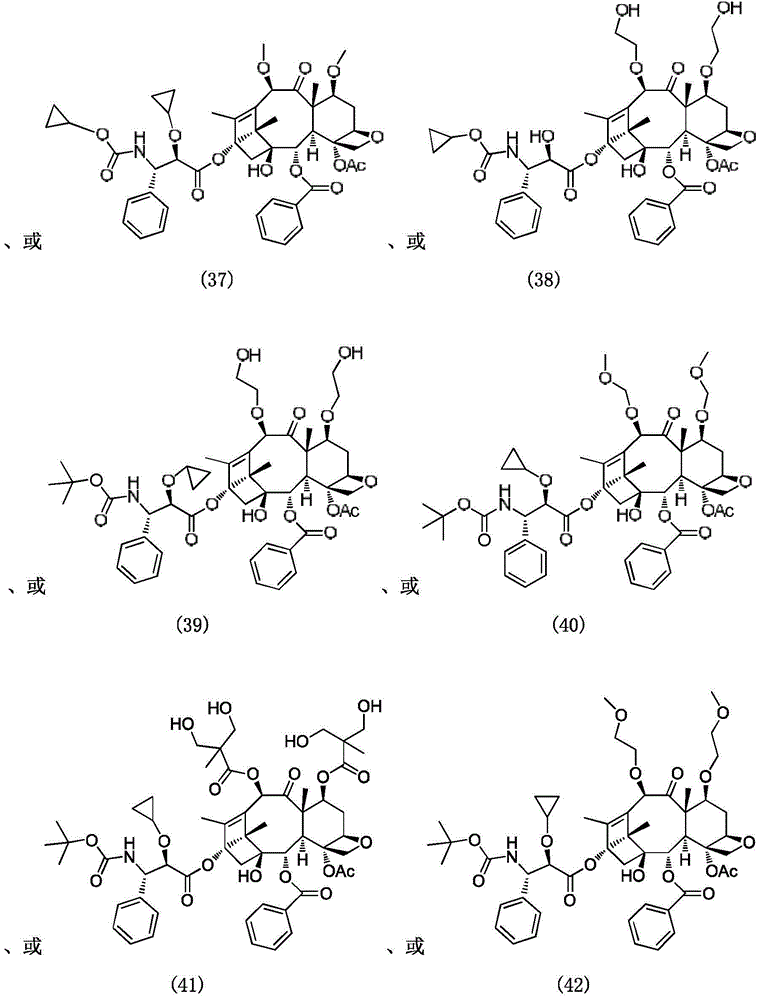
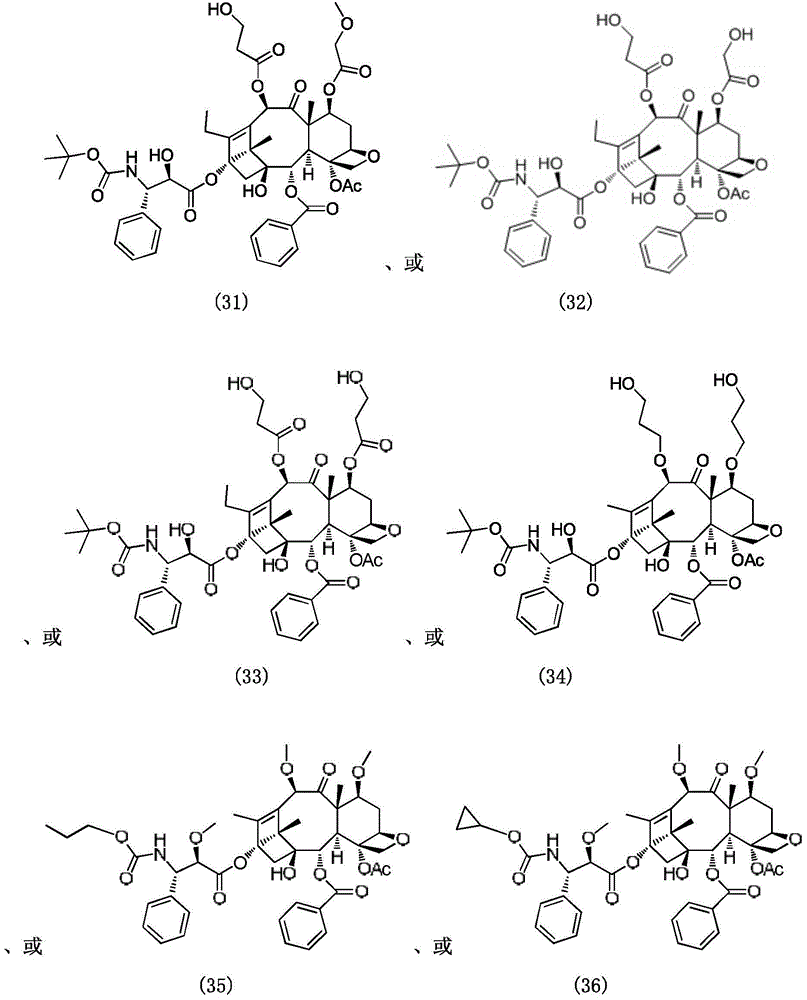
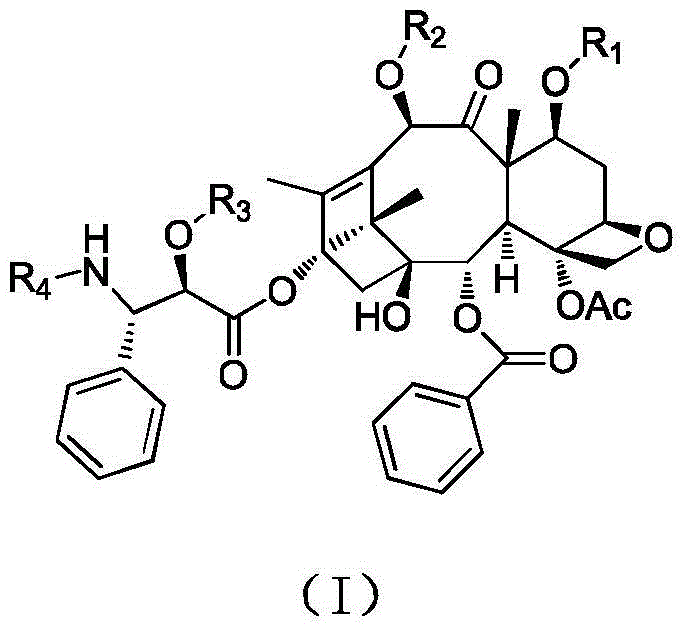
Paclitaxel derivatives and preparation method thereof
A drug and compound technology, applied in the field of paclitaxel derivatives and its preparation, can solve the problems of cardiovascular toxicity, poor water solubility, neurotoxicity, etc., and achieve good anticancer effect and good anticancer activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Synthesis of Example 1 Compound 56
[0042] Under argon protection, TrocCl (2.49ml, 18.18mmol) was added dropwise to a solution of compound 55 (3g, 5.51mmol) in pyridine (54ml) at 0°C, and reacted at room temperature for 1h. TLC detected that the reaction was complete, and pyridine was removed under reduced pressure. Add 80ml ethyl acetate, 50ml water, extract with ethyl acetate (50ml×2), wash with saturated sodium chloride (50ml), dry over anhydrous sodium sulfate, concentrate, column chromatography (PE:EA=3:1) Compound 56 (3.96 g, 81%) was obtained as a white solid.
Embodiment 2
[0043] Synthesis of Example 2 Compound 58
[0044] Add compound 56 (3g, 3.36mmol), compound 57 (3.24g), DCC, and DMAP to the reaction flask in sequence, add 68mL of toluene under the protection of argon, stir and react at 85°C for 2h, after HPLC monitoring the reaction is complete, remove the toluene under reduced pressure , add 80ml of ethyl acetate, 50ml of water, stir for 20min, filter to remove insoluble matter, extract with ethyl acetate (50ml×2), wash with saturated sodium chloride (50ml), dry over anhydrous sodium sulfate, concentrate, column chromatography (PE:EA=3:1) yielded compound 58 (3.70 g, 92%).
Embodiment 3
[0045]Synthesis of Example 3 Compound 59
[0046] Add zinc powder (6.75g, 92.87mmol) and acetic acid to the methanol (120ml) solution of compound 58 (3.7g, 3.09mmol) successively, after reacting at 30 ℃ for 5h, TLC detects that the reaction is complete, the zinc powder is removed by filtration, and the methanol is removed under reduced pressure and acetic acid, add 100ml ethyl acetate, 50ml water, extract with ethyl acetate (50ml×2), wash with saturated sodium chloride (50ml), dry over anhydrous sodium sulfate, concentrate, column chromatography (PE:EA=3 : 1) Compound 59 (2.53 g, 90%) was obtained as a white solid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com