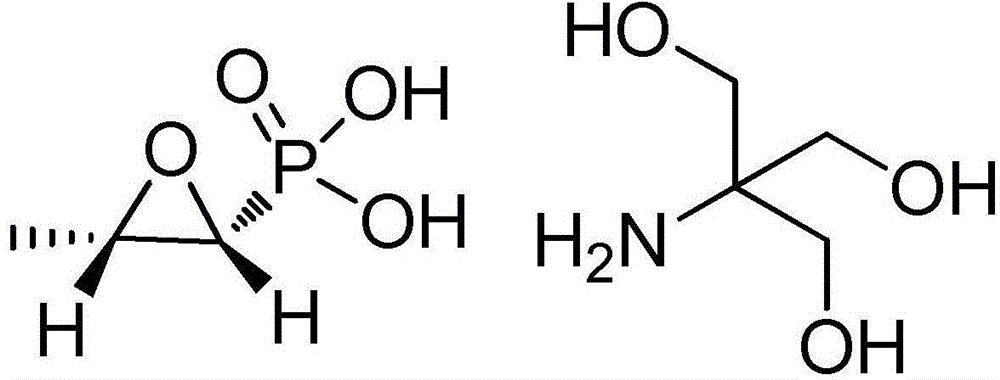
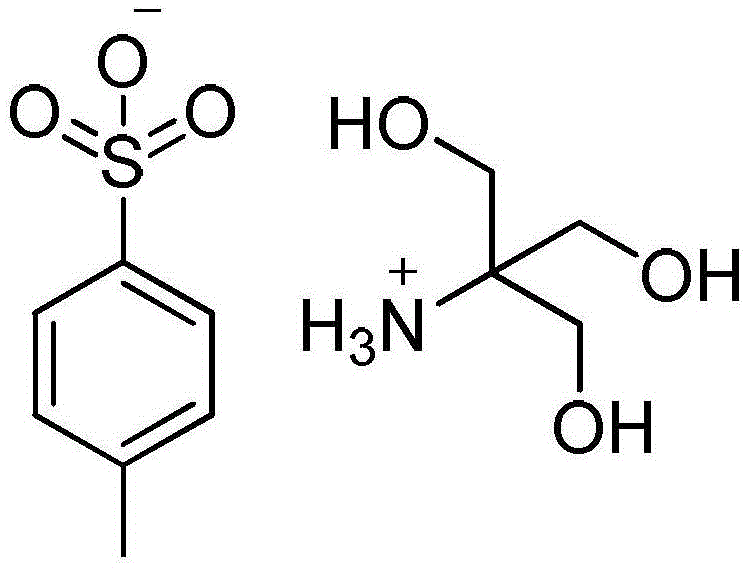
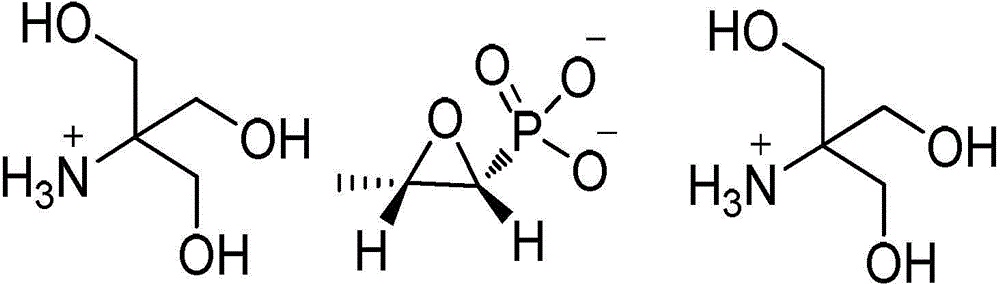
Preparation method of fosfomycin tromethamine
A technology for fosfomycin tromethamine and tromethamine, which is applied in the field of preparation of fosfomycin tromethamine, can solve the problems of high cost, unstable epoxy ring, high cost and the like, and achieves a high stability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (1) Preparation of p-toluenesulfonic acid tromethamine salt: p-toluenesulfonic acid monohydrate and tromethamine are dissolved in dehydrated alcohol, and the mol ratio of p-toluenesulfonic acid monohydrate and tromethamine is 1.0:1.0, the mass of absolute ethanol is 5 times the mass of p-toluenesulfonic acid monohydrate; heat to 70°C, stir until clear and transparent, cool down to -10°C at a rate of 10°C / h, then keep warm for 1h, centrifuge The solid was separated, and the product was washed and filtered with absolute ethanol to obtain a wet product of tromethamine p-toluenesulfonate salt, and the wet product of tromethamine p-toluenesulfonate salt was vacuum-dried at 70°C to obtain a crystal-free Tromethamine p-toluenesulfonate salt of water;
[0034](2) prepare fosfomycin tromethamine crude product: dissolve tromethamine p-toluenesulfonate salt in dehydrated alcohol, and dehydrated alcohol quality is 9 times of tromethamine p-toluenesulfonate salt quality; Stir at 62...
Embodiment 2
[0038] (1) Preparation of p-toluenesulfonic acid tromethamine salt: p-toluenesulfonic acid monohydrate and tromethamine are dissolved in dehydrated alcohol, and the mol ratio of p-toluenesulfonic acid monohydrate and tromethamine is 1.0:1.1, the mass of absolute ethanol is 8 times the mass of p-toluenesulfonic acid monohydrate. Heat to 80°C, stir until clear and transparent, cool down to 0°C at a rate of 8°C / h, then keep warm for 1.5h, centrifuge to obtain the solid, and wash and filter the product with absolute ethanol to obtain tromethamine p-toluenesulfonate Alkoxide wet product, the p-toluenesulfonate tromethamine salt wet product is vacuum-dried at 80°C to obtain the p-toluenesulfonate tromethamine salt without crystal water;
[0039] (2) prepare fosfomycin tromethamine crude product: dissolve tromethamine p-toluenesulfonate in absolute ethanol, and the quality of dehydrated alcohol is 13 times of tromethamine p-toluenesulfonate quality; Stir at 42.5±2.5°C to obtain a cl...
Embodiment 3
[0043] (1) Preparation of p-toluenesulfonic acid tromethamine salt: p-toluenesulfonic acid monohydrate and tromethamine are dissolved in dehydrated alcohol, and the mol ratio of p-toluenesulfonic acid monohydrate and tromethamine is 1.0:1.3, the mass of absolute ethanol is 3 times that of p-toluenesulfonic acid monohydrate. Heat to 60°C, stir until clear and transparent, cool down to 10°C at a rate of 5°C / h, then keep warm for 1 hour, centrifuge to obtain the solid, and wash and filter the product with absolute ethanol to obtain tromethamine p-toluenesulfonate Salt wet product, the p-toluenesulfonate tromethamine salt wet product is vacuum-dried at 60°C to obtain the p-toluenesulfonate tromethamine salt without crystal water;
[0044] (2) Preparation of fosfomycin tromethamine crude product: dissolving p-toluenesulfonate tromethamine salt in dehydrated alcohol, the quality of dehydrated alcohol is 6 times of p-toluenesulfonate tromethamine salt quality; heating Stir at 67.5±2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com