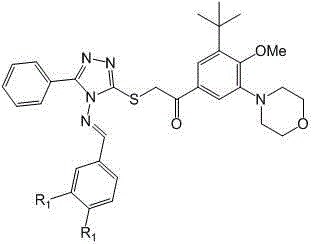
Phenyl triazole schiff base compound as well as preparation method and application thereof
A compound and alkyl technology, applied in the field of drugs related to thrombosis diseases, can solve problems such as high bleeding risk
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] . Reaction raw materials: self-made, conventional method.
[0032] 1.66 g (10 mmol) compound II and 1.92g (10 mmol) of compound III-1 Dissolve in 20 mL of glacial acetic acid and heat to reflux overnight. After the reaction mixture was slightly cooled, it was poured into 200 mL of ice water, stirred, and the solid was collected by suction filtration, recrystallized from absolute ethanol, and vacuum-dried at room temperature to obtain the product IV-1 , white crystals. MS, m / z = 341([M+H] + ).
[0033] 1.70g (5 mmol) compound IV-1 , 1.85 g (5 mmol) compound V and 2.07 g (15 mmol) of solid potassium carbonate were stirred overnight in 15 mL of acetonitrile, and then heated to reflux for 3 hours. The reaction mixture was cooled slightly and poured into 200 mL ice water, stirred, adjusted to pH = 4 with concentrated hydrochloric acid, extracted with 50 mL × 3 dichloromethane, combined organic phases, washed with brine, dried over anhydrous sodium sul...
Embodiment 2-4
[0035] Referring to the method of Example 1, it is possible to synthesize I of the following compounds:
[0036] .
Embodiment 5
[0037] Example 5 In vitro platelet aggregation inhibition test
[0038] Pharmacological tests of substances were performed in TRAP (thrombin receptor activating peptide)-induced platelet aggregation in 96-well plates. 3.13% sodium citrate solution was pre-added in the syringe, and then 20 mL of blood from healthy volunteers was drawn in, at 1500 gPlatelet-rich plasma (PRP) was separated by centrifugation for 20 minutes and treated with 1 μL PGE1 solution (500 μg / mL in ethanol) / mL PRP. After incubation at room temperature for 5 minutes, they were centrifuged at 1200 g for 20 minutes to remove leukocytes. Transfer the leukocyte-free PRP to 15 mL PP tubes in batches at 5 mL / portion, and centrifuge at 3600 g to pellet the platelets. Then, decant the upper plasma layer and resuspend the platelet pellet from 5 mL PRP in 1 mL Tyrode (120 mM NaCl, 2.6 mM KCl, 12 mM NaHCO3, 0.39 mM NaH2PO4, 10 mM HEPES, 0.35% BSA, 5.5 mM Glucose, pH = 7.4) and adjusted to a platelet count of 3 × 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com